La página a la que intenta acceder ha sido trasladada a otra dirección.
https://quibio.web.uah.es/
Por favor, espere a ser redirigido o pulse en el enlace anterior.
The page you are trying to access has been moved to another address.
https://quibio.web.uah.es/
Please wait to be redirected or click on the link above.
https://quibio.web.uah.es/group/
y actualice sus enlaces.
Introducing… Patricia en Angew Chem
Patricia García-García ha publicado su primer Angewandte Chemie como autora de correspondencia:
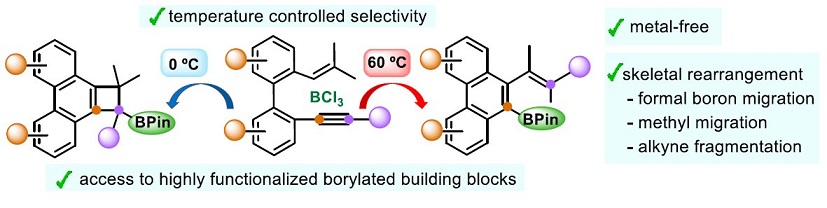
Metal-Free Temperature-Controlled Regiodivergent Borylative Cyclizations of Enynes: BCl3-Promoted Skeletal Rearrangement: A. Milián, M. A. Fernández-Rodríguez, E. Merino, J. J. Vaquero, P. García-García, Angew. Chem. Int. Ed. 2022, doi.org/10.1002/anie.202205651.


 orcid.org/0000-0003-0156-9082
orcid.org/0000-0003-0156-9082