La página a la que intenta acceder ha sido trasladada a otra dirección.
https://quibio.web.uah.es/
Por favor, espere a ser redirigido o pulse en el enlace anterior.
The page you are trying to access has been moved to another address.
https://quibio.web.uah.es/
Please wait to be redirected or click on the link above.
https://quibio.web.uah.es/group/
y actualice sus enlaces.
Publicaciones > Garre et al
Synthesis and Photophysical Behavior of a Highly Fluorescent Family of Unsymmetrical Organoboron Complexes Containing 5-(Pyridin-2-ylmethylene)imidazolidine-2,4-dione Moieties.
1. Departamento de Quimica Organica y Quimica Inorganica, Instituto de Investigacion Quimica "Andres M. del Rio" (IQAR) , Universidad de Alcala, IRYCIS , 28805 Alcala de Henares , Spain. 2. Departamento de Quimica, Centro de Investigacion en Sintesis Quimica (CISQ) , Universidad de La Rioja , Madre de Dios 53 , 26006 Logrono , Spain.
Abstract
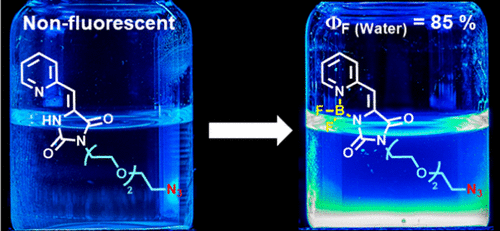
A new and highly fluorescent family of unsymmetrical organoboron complexes containing 5-(pyridin-2-ylmethylene)imidazolidine-2,4-dione moieties has been synthesized in three steps. These compounds show strong absorptions covering a wide range of the UV-vis spectrum and are strongly emissive (varphif of up to 0.92 in CH3CN). Moreover, two fluorophores that include an alkyne or an azide group at the end of the alkyl chain and with potential utility in bioorthogonal chemistry have been developed. One of these, in which the glycol substituent provides an enhanced water solubility without compromising the fluorescence (varphif = 0.85 in water), may be of particular importance.