Synthesis and Photophysical Behavior of a Highly Fluorescent Family of Unsymmetrical Organoboron Complexes Containing 5-(Pyridin-2-ylmethylene)imidazolidine-2,4-dione Moieties
M. Soledad Garre, Raúl Losantos, Sara Gutiérrez, David Sucunza, Patricia García-García, Diego Sampedro and Juan J. Vaquero
J. Org. Chem., Article ASAP
DOI: 10.1021/acs.joc.9b02451
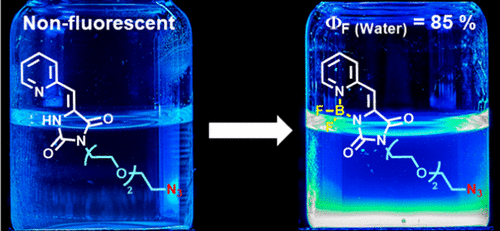
A new and highly fluorescent family of unsymmetrical organoboron complexes containing 5-(pyridin-2-ylmethylene)imidazolidine-2,4-dione moieties has been synthesized in three steps. These compounds show strong absorptions covering a wide range of the UV–vis spectrum and are strongly emissive (ϕf of up to 0.92 in CH3CN). Moreover, two fluorophores that include an alkyne or an azide group at the end of the alkyl chain and with potential utility in bioorthogonal chemistry have been developed. One of these, in which the glycol substituent provides an enhanced water solubility without compromising the fluorescence (ϕf = 0.85 in water), may be of particular importance.
