Publications > Valencia et al
Synthesis of Five-Membered Organoborate Heterocycles via a Metal-Free Carboboration and Their Use in Cross-Coupling Reactions.
Palabras clave: metal-free carboboration; organoboron compounds; organoborate heterocycles; cross-coupling reactions; boron chemistry
Resumen
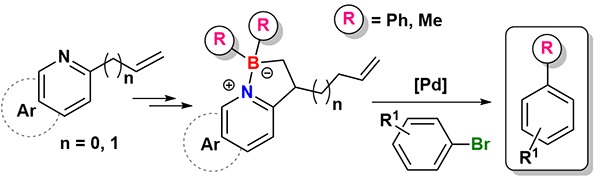
Treatment of various vinylbenzopyridines with allyl(dichloro)borane affords five-membered organoborate heterocycles via a metal-free carboboration. The reaction between these organoborates and Grignard reagents increases the number of derivatives belonging to this novel family of four-coordinate organoboron compounds. Some of them were used as reagents in phenylations and methylations in moderate to high yields under standard palladium-catalyzed cross-coupling reaction conditions.