Metal-Free Temperature-Controlled Regiodivergent Borylative Cyclizations of Enynes: BCl3-Promoted Skeletal Rearrangement
Ana Milián, Manuel A. Fernández-Rodríguez,* Estíbaliz Merino, Juan J. Vaquero, Patricia García-García*
Angew. Chem. Int. Ed. 2022, Accepted Articles
DOI: 10.1002/anie.202205651
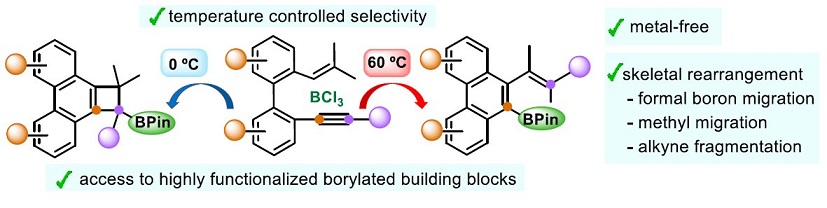
| Metal-free borylative cyclization of biphenyl-embedded 1,3,5-trien-7-ynes in the presence of simple and inexpensive BCl3 provided synthetically useful borylated building blocks. The outcome of the process depends on the reaction temperature, with borylated phenanthrenes obtained at 60 ºC and phenanthrene-fused borylated cyclobutanes formed at 0 ºC. Based on DFT calculations, a mechanism for these novel transformations has been proposed, which involves an uncommon skeletal rearrangement, including migration of a methyl group and alkyne fragmentation, unprecedented in BCl3-promoted cyclization reactions. |