Publications > Baeza et al
Application of Selective Palladium-Mediated Functionalization of the Pyrido%@5B3′,2′:4,5%@5Dpyrrolo%@5B1,2-c%@5Dpyrimidine Heterocyclic System for the Total Synthesis of Variolin B and Deoxyvariolin B
Palabras clave: Cross-coupling; Total synthesis; Natural products; Heterocycles
Resumen
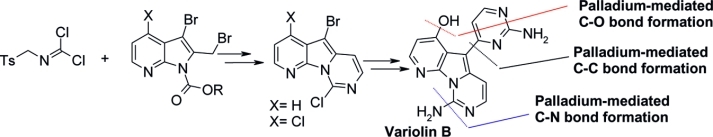
Abstract The reaction of protected 3-bromo-2-(bromomethyl)-4-methoxypyrrolo%@5B2,3-b%@5Dpyridine and tosylmethyl isocyanide (TosMIC) afforded a pyrido%@5B3?,2?:4,5%@5Dpyrrolo%@5B1,2-c%@5Dpyrimidine derivative in good yield. This compound was transformed through installation of the pyrimidine moiety in the C5 position, hydrolysis, and decarboxylation in an advanced intermediate for the total or formal synthesis of the naturalalkaloid variolin B. Reaction of 3-bromo-2-(bromomethyl)-4-chloropyrrolo%@5B2,3-b%@5Dpyridine with N-tosylmethyl dichloroformimide as a synthetic TosMIC equivalent afforded trihalo-substituted pyrido%@5B3?,2?:4,5%@5Dpyrrolo%@5B1,2-c%@5Dpyrimidine. This compound was used in a new total synthesis of the alkaloid variolin B by selective and sequential C?N, C?C, and C?O palladium-mediated functionalization at the C9, C5, and C4 positions of the pyrido%@5B3?,2?:4,5%@5Dpyrrolo%@5B1,2-c%@5Dpyrimidine system. A formal synthesis of deoxyvariolin B is also described by using the same synthetic strategy.