22nd Tetrahedron Symposium
Lisbon, 28 June – 1 July
– Póster:
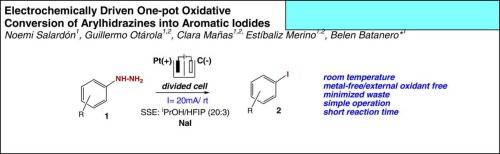
· Photocatalytic synthesis of indazoles from alkynylazobenzenes
Clara Mañas, Estíbaliz Merino
– Póster:
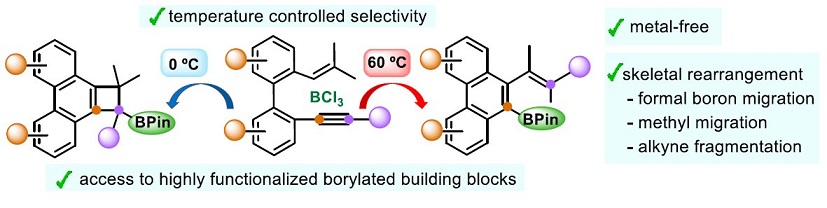
· Metal-free synthesis of medium-sized rings by cationic carbocyclization of trienynes
Jaime Tostado Sánchez, Ana Milián, Juan J. Vaquero, Manuel A. Fernández-Rodríguez
XXXVIII Reunión Bienal de la Real Sociedad Española de Química (RSEQ)
Granada, 27-30 June
– Oral:
· Asymmetric Photocatalysis Through Smiles Rearrangements
Estíbaliz Merino
– Oral:
· Drogas, fármacos y venenos: el impacto de los productos naturales en la historia
David Sucunza
– Póster:
· Progress towards the synthesis of Laetevirenol A
Lucía Sánchez Jiménez, Ana Milián, Jaime Tostado, Juan J. Vaquero, Patricia García García, Manuel A. Fernández Rodríguez
– Póster:
· Gold(I) catalyzed cyclization of alkynylcyclobutanamides towards lactams
Guillermo G. Otárola, María Soledad Garre, Estíbaliz Merino, David Sucunza, Juan J. Vaquero, Patricia García-García
– Póster:
· Catalytic (enantioselective) access to 4-(sec-alkyl)anilines via 1,6-conjugate addition of Grignard reagents to in situ generated aza-p-quinone methides
Mercedes Zurro, L. Ge, S. R. Harutyunyan
XXVIII REUNIÓN BIENAL del Grupo Especializado de Química Orgánica
Granada, 1 July
– Póster:
· Photocatalytic synthesis of a-chloro- and a-hydroxy b-sulfonylamides. Preparation of anticancer drug Bicalutamide
Sergio Torres, Mercedes Zurro, Estíbaliz Merino
– Póster:
· Preparation of 5-aryl-3-trifluoromethylpyrazoles: one-pot metal-free transformation from styrenes and 2,2,2-trifluorodiazoethane
Julia Altarejos, David Sucunza, Juan José Vaquero, Estíbaliz Merino, Javier Carreras