Total Synthesis of Laetevirenol A via Regioselective Gold-Catalyzed and Acid-Promoted Cyclizations
Ana Milián, Lucía Sánchez-Jiménez, Jaime Tostado, Juan J. Vaquero, Manuel A. Fernández-Rodríguez*, Patricia García-García*
Adv. Synth. Catal. 2023, Accepted Articles
DOI: 10.1002/adsc.202301136
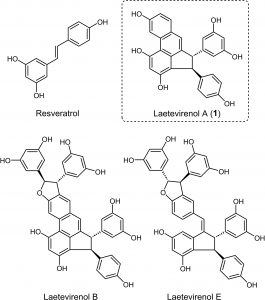
| The total synthesis of Laetevirenol A, a natural product with antioxidant activity, has been achieved. A gold-catalyzed cycloisomerization of an o-alkenyl-o’-alkynylbiphenyl has been used as the key step for the construction of the phenanthrene moiety present in Laetevirenol A. Several studies in model substrates have been carried out to unveil the effect of substituents in different locations in the outcome of this cyclization, which allowed the design of an appropriate precursor for the fundamental gold-catalyzed cycloisomerization. The suitably functionalized phenanthrene intermediate obtained in this key step could be further transformed into Laetevirenol A via a Friedel-Crafts cyclization, which also turned out to be dependent on the nature of the substituents. Finally, Laetevirenol A was obtained in 10 steps from commercially available substrates, with a 20% global yield. |