Publications > de-Lucio et al
Pyridazino-pyrrolo-quinoxalinium salts as highly potent and selective leishmanicidal agents targeting trypanothione reductase.
1. Departamento de Biología de Sistemas, Universidad de Alcalá, E-28805, Alcalá de Henares, Madrid, Spain. Electronic address:. 2. Departamento de Química Orgánica y Química Inorgánica, Universidad de Alcalá, 28805, Alcalá de Henares, Madrid, Spain. 3. Instituto Ramón y Cajal de Investigación Sanitaria (IRYCIS) Ctra, Colmenar Viejo, km. 9100, 28034, Madrid, Spain. 4. Instituto de Investigación Química Andrés Manuel del Río (IQAR), Universidad de Alcalá, 28805, Alcalá de Henares, Madrid, Spain. Electronic address:. 5. Departamento de Química Orgánica y Química Inorgánica, Universidad de Alcalá, 28805, Alcalá de Henares, Madrid, Spain. Electronic address:. 6. Área de Farmacología, Departamento de Ciencias Biomédicas, Unidad Asociada al IQM-CSIC, Universidad de Alcalá, E-28805, Alcalá de Henares, Madrid, Spain. Electronic address:.
a. hector.lucio@edu.uah.es b. javier.garciamarin@uah.es c. sanchezalonsopatricia@gmail.com d. jcarlos.garcias@uah.es e. migueltoro83@gmail.com f. juanjose.vaquero@uah.es g. federico.gago@uah.es h. ramon.alajarin@uah.es i. antonio.jimenez@uah.es
Keywords: Enzyme inhibitor; Leishmania; Pyridazino%@5B2,3-a%@5Dpyrrolo%@5B2,1-c%@5Dquinoxalinium; Trypanothione disulfide reductase
Abstract
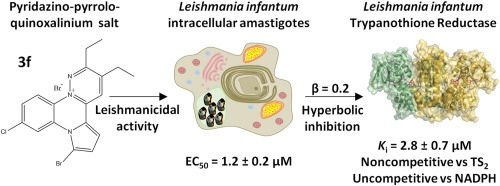
Fifteen pyridazino-pyrrolo-quinoxalinium salts were synthesized and tested for their antiprotozoal activity against Leishmania infantum amastigotes. Eleven of them turned out to be leishmanicidal, with EC(50) values in the nanomolar range, and displayed low toxicity against the human THP-1 cell line. Selectivity indices for these compounds range from 10 to more than 1000. Compounds 3b and 3f behave as potent inhibitors of the oxidoreductase activity of the essential enzyme trypanothione disulfide reductase (TryR). Interestingly, binding of 3f is not affected by high trypanothione concentrations, as revealed by the noncompetitive pattern of inhibition observed when tested in the presence of increasing concentrations of this substrate. Furthermore, when analyzed at varying NADPH concentrations, the characteristic pattern of hyperbolic uncompetitive inhibition supports the view that binding of NADPH to TryR is a prerequisite for inhibitor-protein association. Similar to other TryR uncompetitive inhibitors for NADPH, 3f is responsible for TryR-dependent reduction of cytochrome c in a reaction that is typically inhibited by superoxide dismutase.