Publications > Garcia-Marin et al
Tripeptides as Integrin-Linked Kinase Modulating Agents Based on a Protein-Protein Interaction with α-Parvin.
1. Departamento de Química Orgánica y Química Inorgánica, Universidad de Alcalá, Alcalá de Henares 28805, Spain. 2. Instituto Ramón y Cajal de Investigación Sanitaria (IRYCIS), Ctra. Colmenar Viejo, km. 9100, Madrid 28034, Spain. 3. Instituto de Investigación Química Andrés Manuel del Río (IQAR), Universidad de Alcalá, Alcalá de Henares 28805, Spain. 4. Departamento de Biología de Sistemas, Universidad de Alcalá, Alcalá de Henares 28805, Spain. 5. Graphenano Medical Care, S.L, Yecla 30510, Spain.
Abstract
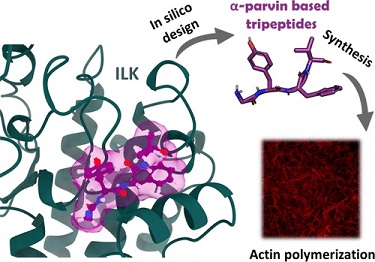
Integrin-linked kinase (ILK) has emerged as a controversial pseudokinase protein that plays a crucial role in the signaling process initiated by integrin-mediated signaling. However, ILK also exhibits a scaffolding protein function inside cells, controlling cytoskeletal dynamics, and has been related to non-neoplastic diseases such as chronic kidney disease (CKD). Although this protein always acts as a heterotrimeric complex bound to PINCH and parvin adaptor proteins, the role of parvin proteins is currently not well understood. Using in silico approaches for the design, we have generated and prepared a set of new tripeptides mimicking an α-parvin segment. These derivatives exhibit activity in phenotypic assays in an ILK-dependent manner without altering kinase activity, thus allowing the generation of new chemical probes and drug candidates with interesting ILK-modulating activities.