Publications > Hu et al
Asymmetric, Remote C(sp(3) )-H Arylation via Sulfinyl-Smiles Rearrangement.
1. Department of Chemistry, University of Zurich, Winterthurerstrasse 190, CH 8057, Zurich, Switzerland. 2. Departamento de Química Orgánica y Química Inorgánica Instituto de Investigación Química "Andrés M. del Río" (IQAR). Facultad de Farmacia, Universidad de Alcalá Alcalá de Henares, 28805, Madrid, Spain. 3. Instituto Ramón y Cajal de Investigación Sanitaria (IRYCIS), Ctra. de Colmenar Viejo, Km. 9.100, 28034, Madrid, Spain.
Keywords: Asymmetric Remote Arylation; Hydrogen Atom Transfer; Photoredox Catalysis; Sulfinyl-Smiles Rearrangement; α-Arylated Amides
Abstract
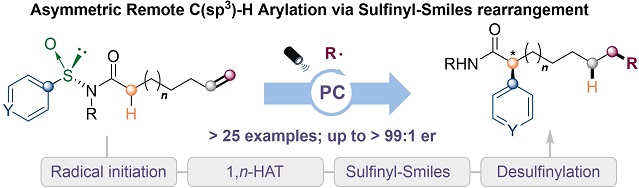
An efficient asymmetric remote arylation of C(sp(3) )-H bonds under photoredox conditions is described here. The reaction features the addition radicals to a double bond followed by a site-selective radical translocation (1,n-hydrogen atom transfer) as well as a stereocontrolled aryl migration via sulfinyl-Smiles rearrangement furnishing a wide range of chiral α-arylated amides with up to >99 : 1 er. Mechanistic studies indicate that the sulfinamide group governs the stereochemistry of the product with the aryl migration being the rate determining step preceded by a kinetically favored 1,n-HAT process.