Regiodivergent electrophilic cyclizations of alkynylcyclobutanes for the synthesis of cyclobutane-fused O-heterocycles
María Soledad Garre, David Sucunza, Enrique Aguilar, Patricia García-García, and Juan J. Vaquero
J. Org. Chem., Article ASAP
DOI: 10.1021/acs.joc.9b00618
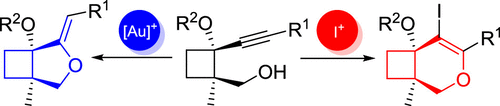
Cyclobutane-fused dihydropyrans and methylenetetrahydrofurans are highly interesting cores found in numerous natural products. Both these cores are selectively prepared from a common alkynylcyclobutane precursor bearing an appended hydroxyl group herein. Thus, cyclobutane-fused dihydropyrans can be obtained by a selective 6-endo-dig iodocyclization, whereas gold-catalyzed 5-exo-dig cycloisomerization provides the bicyclic core containing a methylenetetrahydrofuran moiety as major product. Several cyclobutane-fused O-heterocycles with diverse substitution are synthesized following the reported methodology.
