Publications > Salardón et al
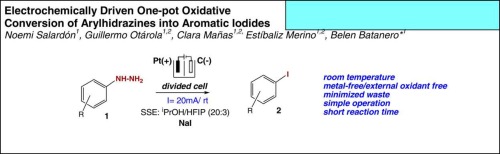
Electrochemically driven one-pot oxidative conversion of arylhydrazines into aromatic iodides.
Palabras clave: Environmentally friendly transformation; Aryl iodides; Iodide redox mediator; Arylhydrazines; Anodic oxidation; C-N bond cleavage; Electrografting
Resumen
The efficient metal-free electrosynthesis of 2,4-dinitrophenyl iodide is here reported starting from 2,4-dinitrophenylhydrazine. Surprisingly this dinitrated arylhydrazine minimizes, under the applied experimental conditions, any anodic multilayered film formation. This sustainable iodide-mediated oxidative dehydrazination enables coupling reaction of electrogenerated iodine with aryl radicals from electron-deficient arylhydrazines employing electricity as the driving force and an inexpensive halogen source. A mechanistic proposal explaining the formation of aryl iodides is presented and discussed.