Publications > Sánchez-Alonso et al
Pyrrolo[1,2-a]quinoxal-5-inium salts and 4,5-dihydropyrrolo[1,2-a]quinoxalines: Synthesis, activity and computational docking for protein tyrosine phosphatase 1B.
1. Departamento de Química Orgánica y Química Inorgánica, Universidad de Alcalá, 28805 Alcalá de Henares, Madrid, Spain. 2. Graphenano Medical Care, SL, Spain. 3. Instituto Ramón y Cajal de Investigación Sanitaria (IRYCIS), Ctra. Colmenar Viejo, km. 9100, 28034 Madrid, Spain. 4. Instituto de Investigación Química Andrés M. del Río (IQAR), Facultad de Farmacia, Universidad de Alcalá, 28805 Alcalá de Henares, Madrid, Spain. 5. Instituto Ramón y Cajal de Investigación Sanitaria (IRYCIS), Ctra. Colmenar Viejo, km. 9100, 28034 Madrid, Spain. 6. Departamento de Biología de Sistemas, Universidad de Alcalá, 28805 Alcalá de Henares, Madrid, Spain. 7. Fundación Renal Iñigo Álvarez de Toledo (FRIAT) y REDinREN del Instituto de Salud Carlos III, Madrid, Spain. 8. Instituto de Investigación Química Andrés M. del Río (IQAR), Facultad de Farmacia, Universidad de Alcalá, 28805 Alcalá de Henares, Madrid, Spain. Electronic address:.
a. ramon.alajarin@uah.es
Keywords: 4,5-Dihydropyrrolo[1,2-a]quinoxaline, pyrrolo[1,2-a]quinoxal-5-inium; Diabetes mellitus; Inhibitor; PTP1B; Phosphatase; Selectivity; TC-PTP
Abstract
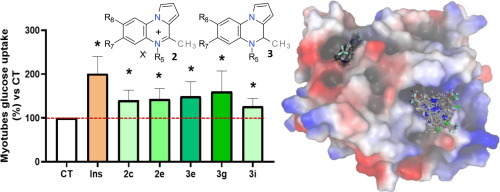
Protein tyrosine phosphatase (PTP1B) is an interesting therapeutical target for diabetes, obesity, heart disease and cancer. As such, inhibition of PTP1B using orally administered drugs is still being pursued by academia and pharmaceutical companies. The failure of catalytic-site inhibitors led to the focus in this field being switched to allosteric inhibitors. To date, the non-competitive inhibitors that have reached clinical trials target the site formed by the α3/α6/α7 tunnel or the site found in a disordered C-terminal non-catalytic segment. Herein, pyrrolo[1,2-a]quinoxal-5-inium salts and 4,5-dihydropyrrolo[1,2-a]quinoxalines are synthesized from pyrrolo[1,2-a]quinoxalines by alkylation and reduction, respectively. These compounds showed no toxicity in HepG2 cells and exhibited inhibitory activity against PTP1B, with inhibition percentages of between 37% and 53% at 1 μM and activities (IC(50)) of between 0.25 and 1.90 μM. The inhibitory activity against T-cell protein tyrosine phosphatase (TC-TPT) was also assayed, with 4,5-dihydropyrrolo[1,2-a]quinoxalines being found to be slightly more active and selective. Compounds from the two series behave as insulin mimetics since they exhibit enhancement of glucose uptake in C2C12 cells. Computational docking studies provide information about the putative binding mode for both series and the preference for the α3/α6/α7 allosteric tunnel.