https://quibio.web.uah.es/group/
y actualice sus enlaces.
Publicaciones > Abarca et al
Efficient synthesis of an indoloquinolizinium alkaloid selective DNA-binder by ring-closing metathesis.
Departamento de Quimica Organica y Quimica Inorganica, double daggerDepartamento de Biologia de Sistemas, section signDepartamento de Quimica Analitica, Quimica Fisica e Ingenieria Quimica, Universidad de Alcala , 28871-Alcala de Henares, Madrid, Spain.
Abstract
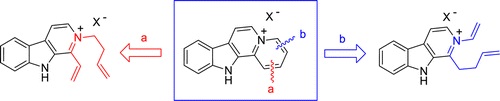
Two total syntheses of the indolo[2,3-a]quinolizinium cation have been accomplished through the application of two ring-closing metathesis reactions to form the pyridinium ring. One of these approaches provides the tetracyclic cation in only five steps from commercially available harmane. Fluorescence-based thermal denaturation experiments, as well as spectrofluorimetric titration, circular dichroism measurements, and theoretical simulations, showed a consistent DNA-binding capacity by intercalation with a marked preference for AT-rich sequences.