https://quibio.web.uah.es/group/
y actualice sus enlaces.
Publicaciones > Abengozar et al
C-H Functionalization of BN-Aromatics Promoted by Addition of Organolithium Compounds to the Boron Atom.
1. Departamento de Quimica Organica y Quimica Inorganica, Instituto de Investigacion Quimica "Andres M. del Rio" (IQAR) , Universidad de Alcala , 28805 Alcala de Henares , Madrid , Spain. 2. Departamento de Quimica Analitica, Quimica Fisica e Ingenieria Quimica, Instituto de Investigacion Quimica "Andres M. del Rio" (IQAR) , Universidad de Alcala , 28805 Alcala de Henares , Madrid , Spain. 3. Centro de Espectroscopia de Resonancia Magnetica Nuclear (CERMN), CAI Quimicas , Universidad de Alcala , 28805 Alcala de Henares , Madrid , Spain.
Abstract
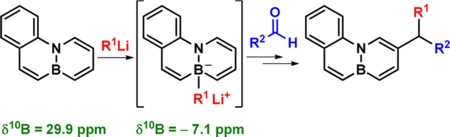
Addition of an organolithium compound to a BN-phenanthrene with embedded B and N atoms is proposed to result in coordination of RLi to the boron atom. This coordination, supported by NMR spectroscopy and DFT calculations, increases the nucleophilicity of the system in the beta position to the N atom and is therefore a useful tool for promoting regioselective C-H functionalization of BN aromatics.