https://quibio.web.uah.es/group/
y actualice sus enlaces.
Highly efficient unbridged D-A+(D) chromophores based on the quinolizinium cation for nonlinear optical (NLO) applications
Esmeralda Sánchez-Pavón, Javier Recio, Marco Antonio Ramirez, Belen Batanero, Koen Clays, Francisco Mendicuti, Gema Marcelo, Thais Carmona, Obis Castaño, Silvia Angelova, Jose L. Andres, Juan J. Vaquero, Ana M. Cuadro*
Dyes and Pigments 2022, ASAP
DOI: 10.1016/j.dyepig.2022.110323
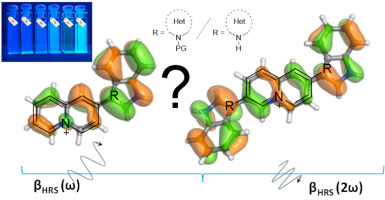
| Novel charged D-A+ chromophores based on quinolizinium cations as acceptor unit have been prepared by treating haloquinolizinium salts with N-heteroarylstannanes under Stille reaction conditions. This approach provides an easy access to potential one-dimensional D-A+ and two-dimensional D-A+-D chromophores in which the acceptor moiety (A+) is the simple azonia cation and the donors are different π-rich N-heterocycles. The first hyperpolarizabilities (β) were measured by hyper-Rayleigh scattering experiments and the experimental data confirmed that the inherent polarization between donor and acceptor fragments modulates the NLO properties. The electronic structures and properties (including both the linear and nonlinear optical properties) of the quinolizinium chromophores were examined by theoretical (DFT, HF and MP2) calculations. A promising strategy for the rational design of D-A building blocks to create new organic-based NLO materials is proposed.. |