La página a la que intenta acceder ha sido trasladada a otra dirección.
https://quibio.web.uah.es/
Por favor, espere a ser redirigido o pulse en el enlace anterior.
The page you are trying to access has been moved to another address.
https://quibio.web.uah.es/
Please wait to be redirected or click on the link above.
Esta versión de nuestra web ya no se mantiene actualizada.
¡Gracias!
Organización XVII SIJ-RSEQ

Varios componentes del grupo se han encargado de organizar el XVII Simposio de Investigadores Jóvenes de la RSEQ, celebrado en Alcalá de Henares del 23 al 26 de noviembre.
XVII Simposio de Investigadores Jóvenes de la RSEQ
Además, ha contribuido con diversas contribuciones:
– Oral:
· Synthesis of seven- and eight-membered rings by Brønsted acid-catalyzed cyclization of biphenyl embedded trienynes
Jaime Tostado Sánchez, Juan J. Vaquero, Manuel A. Fernández-Rodríguez
– Flash:
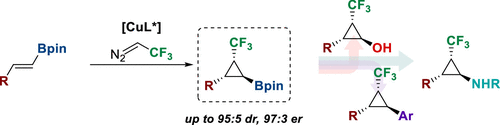
· Enantio- and diastereoselective cyclopropanation of trans-alkenylboronates: Synthesis of versatile (trifluoromethyl)cyclopropylboronates
Julia Altarejos, David Sucunza, Juan José Vaquero, Javier Carreras
– Poster:
· Synthesis of a series of novel bicyclic amino acids
Álvaro González, Francisco José Torrero, José Luis Aceña, Juan José Vaquero
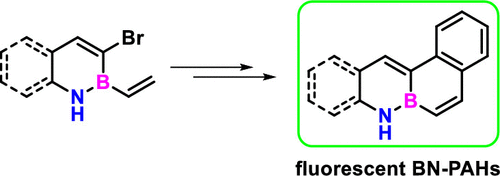
· Metal-free straightforward synthesis of boron-functionalized indenes and fulvenes by borylative cyclization
Ester Sans Panadés, Patricia García-García, Juan J. Vaquero, Cintia Virumbrales, Roberto Sanz, Manuel A. Fernández-Rodríguez
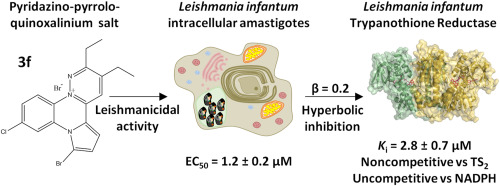
· SYNTHESIS OF PEPTIDE NUCLEIC ACIDS (PNAs) FOR THE TREATMENT OF CHRONIC KIDNEY DISEASE
Francisco Maqueda Zelaya, Verónica Miguel, José Luis Aceña, Santiago Lamas, Juan José Vaquero
· Synthesis of 4-membered rings fused lactams by gold(I) catalyzed
cyclization of alkynylcyclobutanamides
Guillermo G. Otárola, María Soledad Garre, Estíbaliz Merino, David Sucunza, Juan J. Vaquero, Patricia García-García
· Regioselective Ir-Catalyzed C−H Borylation of 4a,8a-Dihydro-4a-Aza-8a-Boranaphthalene
Isabel Valencia, Patricia García, David Sucunza, Juan J. Vaquero
· DEVELOPMENT OF A SYNTHETIC ROUTE TO LAETEVIRENOL A
Lucía Sánchez-Jiménez, Ana Milián, Jaime Tostado, Juan J. Vaquero, Patricia García-García, Manuel A. Fernández-Rodríguez
· Kinetic resolution in transannular Morita-Baylis-Hillman reaction:
approach to the synthesis of guaiane-type sesquiterpenes
Rubén Manzano, Raquel Mato, Efraím Reyes, Liher Prieto, Uxue Uria, Luisa Carrillo, Jose L. Vicario
· Electrochemical synthesis of aryl iodides from arylhydrazines
Clara Mañas Hernández, Guillermo Otárola, Estíbaliz Merino, Noemi Salardón, Belén Batanero