https://quibio.web.uah.es/group/
y actualice sus enlaces.
Publicaciones > García-Marín et al
Insight into the mechanism of molecular recognition between human Integrin-Linked Kinase and Cpd22 and its implication at atomic level.
1. Departamento de Química Orgánica y Química Inorgánica, Instituto de Investigación Química Andrés M. del Río (IQAR), Universidad de Alcalá (IRYCIS), Alcalá de Henares, 28805, Madrid, Spain. 2. Departamento de Química Biológica y Estructural, Centro de Investigaciones Biológicas, CIB-CSIC, C/Ramiro de Maeztu 9, 28040, Madrid, Spain. 3. Department of Chemistry, University of Bath, Claverton Down, Bath, BA2 7AX, UK.
a. javier.garciamarin@uah.es b. javier.garciamarin@uah.es c. javier.garciamarin@uah.es
Keywords: Cpd22; ILK; Integrin-linked kinase; Molecular dynamics; PELE; Pseudokinase
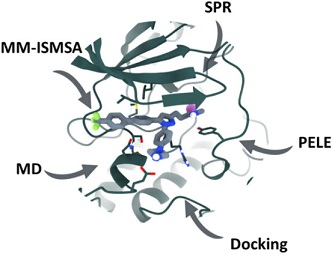
Abstract
Pseudokinases have received increasing attention over the past decade because of their role in different physiological phenomena. Although pseudokinases lack several active-site residues, thereby hindering their catalytic activity, recent discoveries have shown that these proteins can play a role in intracellular signaling thanks to their non-catalytic functions. Integrin-linked kinase (ILK) was discovered more than two decades ago and was subsequently validated as a promising target for neoplastic diseases. Since then, only a few small-molecule inhibitors have been described, with the V-shaped pyrazole Cpd22 being the most interesting and characterized. However, little is known about its detailed mechanism of action at atomic level. In this study, using a combination of computational chemistry methods including PELE calculations, docking, molecular dynamics and experimental surface plasmon resonance, we were able to prove the direct binding of this molecule to ILK, thus providing the basis of its molecular recognition by the protein and the effect over its architecture. Our breakthroughs show that Cpd22 binding stabilizes the ILK domain by binding to the pseudo-active site in a similar way to the ATP, possibly modulating its scaffolding properties as pseudokinase. Moreover, our results explain the experimental observations obtained during Cpd22 development, thus paving the way to the development of new chemical probes and potential drugs.