Esta versión de nuestra web ya no se mantiene actualizada.
Por favor, visite nuestra web operativa en
https://quibio.web.uah.es/group/
y actualice sus enlaces.
https://quibio.web.uah.es/group/
y actualice sus enlaces.
¡Gracias!
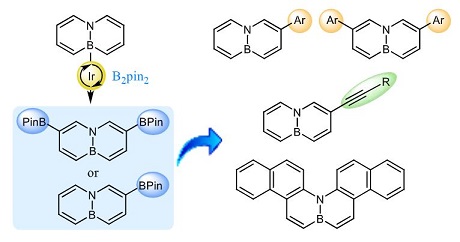
β-Functionalization of 4a-aza-8a-boranaphthalene via Iridium-catalyzed C−H Borylation
Isabel Valencia, David Sucunza, Francisco Mendicuti, Patricia García-García,* Juan J. Vaquero
Adv. Synth. Cat. 2023, Accepted Articles
DOI: 10.1002/adsc.202300408
| A general method for the functionalization of 4a-aza-8a-boranaphthalene in the position β to the nitrogen atom has been developed. This method is based on a regioselective iridium-catalyzed C−H activation process for the introduction of a boronate group, which can subsequently be transformed into a variety of aryl or alkynyl groups via cross-coupling reactions. Selective mono- or difunctionalization can be achieved by controlling the reaction conditions during the borylation step. The photophysical properties of the obtained 3- or 3,6-substituted BN-naphthalenes have been evaluated, and some of them have been found to be significantly fluorescent, with fluorescence quantum yields up to 0.85. |