Esta versión de nuestra web ya no se mantiene actualizada.
Por favor, visite nuestra web operativa en
https://quibio.web.uah.es/group/
y actualice sus enlaces.
https://quibio.web.uah.es/group/
y actualice sus enlaces.
¡Gracias!
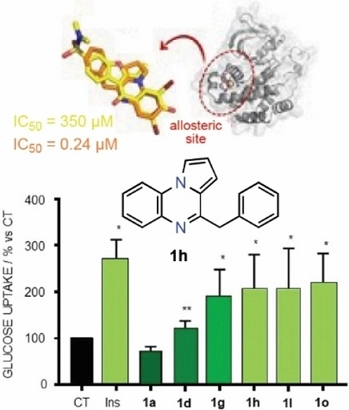
Pyrrolo[1,2‐a]quinoxalines: Insulin Mimetics that Exhibit Potent and Selective Inhibition against Protein Tyrosine Phosphatase 1B
Javier García‐Marín, Mercedes Griera, Patricia Sánchez‐Alonso, Bruno Di Geronimo, Francisco Mendicuti, Manuel Rodríguez‐Puyol*, Ramón Alajarín*, Beatriz de Pascual‐Teresa, Juan J. Vaquero*, Diego Rodríguez‐Puyol*
ChemMedChem, 2020, Early View
DOI: 10.1002/cmdc.202000446
Highlighted as Cover Picture: link
| PTP1B dephosphorylates insulin receptor and substrates to modulate glucose metabolism. This enzyme is a validated therapeutic target for type 2 diabetes, but no current drug candidates have completed clinical trials. Pyrrolo[1,2‐a]quinoxalines substituted at positions C1–C4 and/or C7–C8 were found to be nontoxic to cells and good inhibitors in the low‐ to sub‐micromolar range, with the 4‐benzyl derivative being the most potent inhibitor (0.24 μm). Some analogues bearing chlorine atoms at C7 and/or C8 kept potency and showed good selectivity compared to TCPTP (selectivity index >40). The most potent inhibitors behaved as insulin mimetics by increasing glucose uptake. The 4‐benzyl derivative inhibited insulin receptor substrate 1 and AKT phosphorylation. Molecular docking and molecular dynamics simulations supported a putative binding mode for these compounds to the allosteric α3/α6/α7 pocket, but inconsistent results in enzyme inhibition kinetics were obtained due to the high tendency of these inhibitors to form stable aggregates. Computational calculations supported the druggability of inhibitors. |