https://quibio.web.uah.es/group/
y actualice sus enlaces.
A New Member of the BN-Phenanthrene Family: Understanding the Role of the B—N Bond Position
Alberto Abengózar, David Sucunza, Patricia García-García, Diego Sampedro, Adrián Pérez-Redondo, and Juan J. Vaquero
J. Org. Chem., Article ASAP
DOI: 10.1021/acs.joc.9b00800
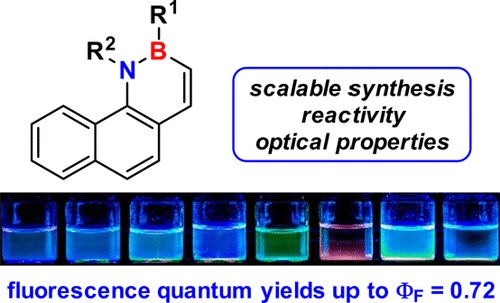
3,4-Dihydro-4-aza-3-boraphenanthrene, which shows the highest fluorescence quantum yield of all nonsubstituted BN-phenanthrenes reported to date (ϕF = 0.61), has been synthesized in only three steps (76% overall yield) from easily accessible 1-bromo-2-vinylnaphthalene, along with several substituted derivatives. The reactivity of these previously unknown BN-aromatic compounds toward organolithium compounds and bromine has been studied. This latter reaction affords bromo-substituted compounds that are suitable for further functionalization via Suzuki and Sonogashira couplings, with complete regioselectivity. The optical properties and excited state deactivation mechanisms of selected compounds were studied using computational methods.
