Enantioselective copper(II) catalysed (4 + 1) cycloaddition of aza-o-quinone methides and bromomalonates. Facile access to enantioenriched indolines
Sergio Torres-Oya, Manuel A. Fernández-Rodríguez* and Mercedes Zurro*
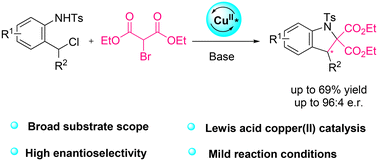
Optically active indolines are valuable structural motifs present in numerous naturally occurring and biologically active molecules. Although several methodologies have been reported in the literature for the synthesis of chiral indolines, many of them rely on the hydrogenation of indoles using expensive metal catalysts. In this report, a copper(II)-catalysed enantioselective (4 + 1) cycloaddition of aza-o-quinone methides (aza-o-QMs) with bromomalonates to access indolines is described. The reactive aza-o-QMs are generated in situ from simple and easily accessible 2-chloromethyl arylsulfonamides under basic conditions, and subsequently undergo cyclization with the in situ formed bromomalonate anion to deliver diverse chiral indoline derivatives in up to 69% yields and 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 er. Scale up and further derivatizations occurred without erosion of enantioselectivity, showing the robustness of this methodology.
4 er. Scale up and further derivatizations occurred without erosion of enantioselectivity, showing the robustness of this methodology.
Org. Biomol. Chem. 2026
DOI: https://doi.org/10.1039/D6OB00091F
