Publications > Abengozar et al
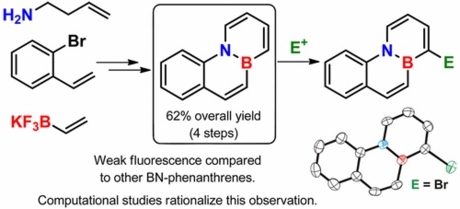
Synthesis, Optical Properties, and Regioselective Functionalization of 4a-Aza-10a-boraphenanthrene.
1. Departamento de Quimica Organica y Quimica Inorganica, Universidad de Alcala , 28871 Alcala de Henares, Madrid, Spain. 2. Departamento de Quimica Analitica, Quimica Fisica e Ingenieria Quimica, Universidad de Alcala , 28871 Alcala de Henares, Madrid, Spain. 3. Departamento de Quimica, Centro de Investigacion en Sintesis Quimica (CISQ), Universidad de La Rioja , Madre de Dios 53, 26006 Logrono, Spain.
Abstract
4a-Aza-10a-boraphenanthrene has been synthesized in only four steps from commercially available materials with a remarkable overall yield of 62%. In contrast to other BN-isosteres of phenathrene, this isomer is weakly fluorescent, which has been explained by means of computational studies that found a low energy conical intersection for the nonradiative deactivation of the excited state. Moreover, a completely regioselective functionalization of 4a-aza-10a-boraphenanthrene at C-1 by reaction with activated electrophiles has been achieved.