Synthesis of Polysubstituted Naphthalenes via Metal-Free Borylative Cyclization of o-Alkynylstyrenes
Marcos Humanes, Manuel A. Fernández-Rodríguez,* Patricia García-García*
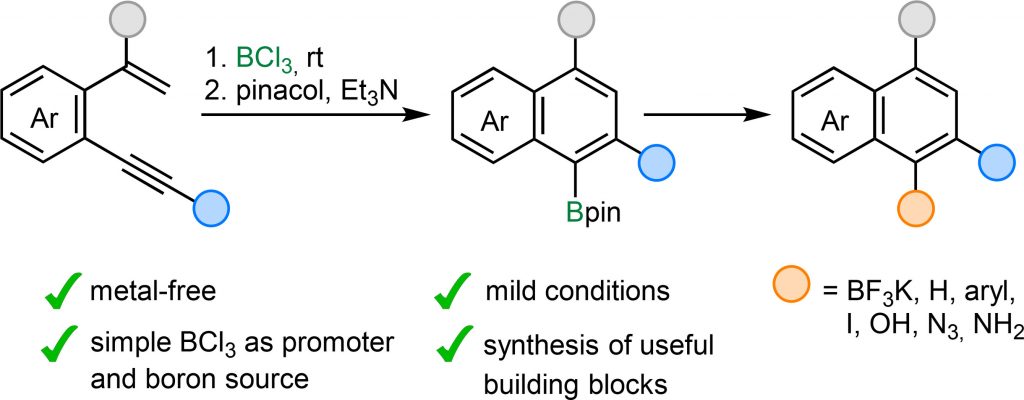
A selective, metal-free method for the synthesis of boron-functionalized polysubstituted naphthalenes via BCl3-mediated cyclization of α-substituted o-alkynylstyrenes is described. The reaction exhibits broad substrate scope, tolerating various groups such as ethers, sulfides and halogens, and delivers high yields under mild conditions, even at gram scale. The resulting Bpin-functionalized naphthalenes serve as versatile building blocks, facilitating further transformations of the C−B bond into C−H, C−C, C−I, C−O, and C−N bonds, thereby increasing molecular complexity and enabling the design of more functionalized products. Notably, this sustainable and straightforward protocol selectively introduces the Bpin group at the sterically hindered α-carbon of the naphthalene framework, complementing C–H borylations of the naphthalene core, that preferentially occur at the β-position. As a result, the developed approach significantly broadens the accessibility of B-functionalized naphthalene derivatives for synthetic and application-oriented purposes.
Adv. Synth. Cat. 2025
DOI: 10.1002/adsc.202500244