Our group was well-represented at the XL Meeting of Chemistry of the RSEQ in Bilbao! We’re proud to announce that our team presented posters, flash communications, and two oral communications, sharing our latest research.

– Oral:
· Regiodivergent transformations of alkynylazobenzenes
Estíbaliz Merino

· Visible-Light Mediated Phenylalanine Modification in Oligopeptides by Debenzylation/Amination Process
Guillermo Morales-Ortega, Iván Pérez, Enrique Gómez-Bengoa, Arkaitz Correa, Javier Carreras

– Flash:
· Acid-mediated cationic cyclization of o-acryloyl-o’-alkynylbiaryls for the synthesis of fluorescent functionalized polycycles
Alex Hipólito-Barriuso, Jaime Mateos, Patricia García-García, Manuel A. Fernández-Rodríguez

– Poster:
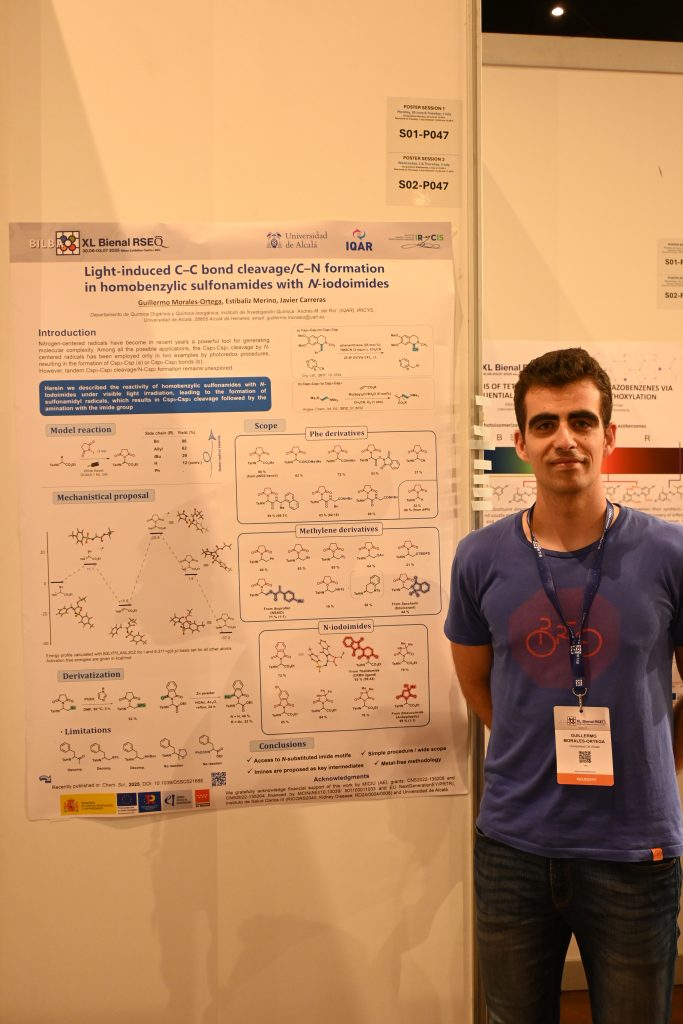
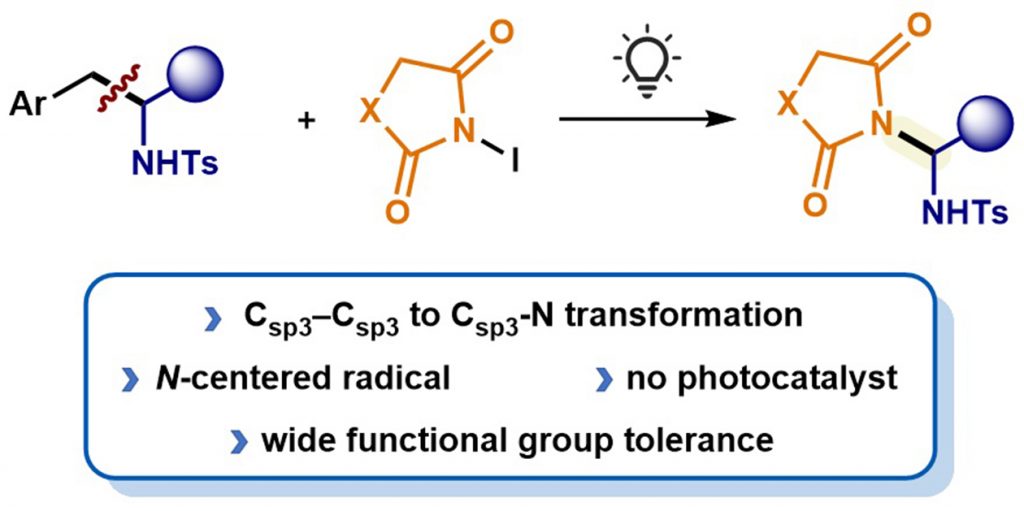
· Light-induced C–C bond cleavage/C–N formation in homobenzylic
sulfonamides with N-iodoimides
Guillermo Morales-Ortega, Estíbaliz Merino, Javier Carreras
– Poster:
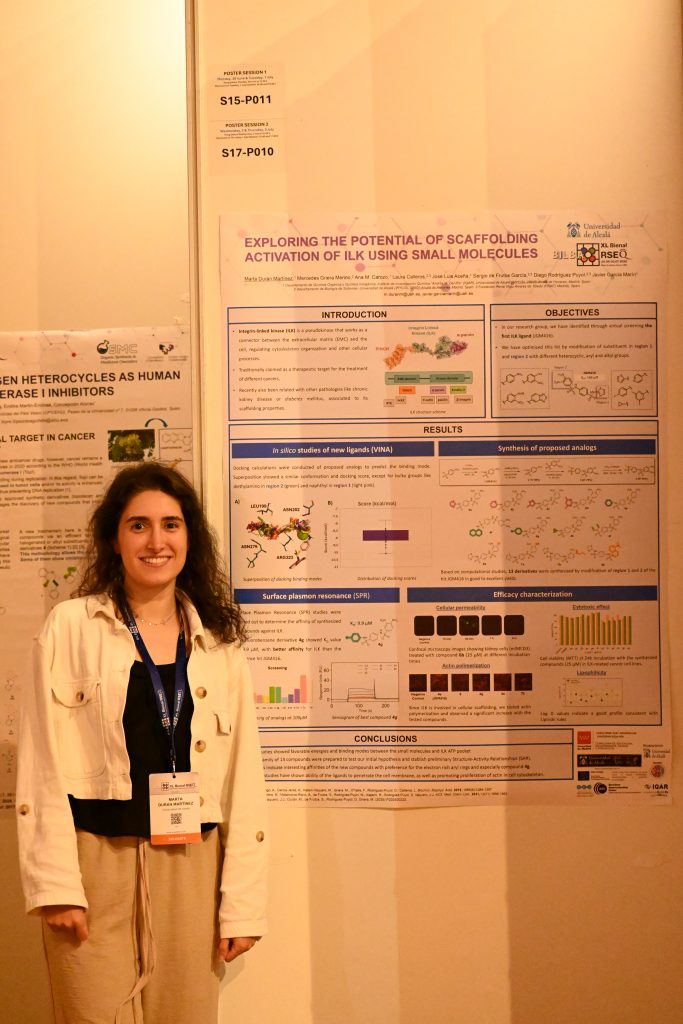
· Exploring the potential of scaffolding activation of ilk using small molecules
Marta Durán Martínez, Mercedes Griera Merino, José Luis Aceña,1 Laura Calleros, Sergio de Frutos García, Diego Rodríguez Puyol, Javier García Marín
– Poster:
· Synthesis of 2,4,6-trisubstituted pyridines by Heterocyclization of TosMIC Derivatives
Jose Antonio García-García, Jose Luis Aceña Bonilla, David Sucunza.

– Poster:
· Enantioselective copper (ii) catalysed (4+1) cycloaddition of Aza-o-quinone methides and bromomalonates: facile access to chiral Indolines
Sergio Torres-Oya, Manuel A. Fernández-Rodríguez, Mercedes Zurro














 orcid.org/
orcid.org/