Our two young members Guillermo Sánchez Mateos and Erica Salivioli have been awarded with a FPI-UAH contract to carry out their doctoral thesis at the UAH.
Congratulations 😊!
Our two young members Guillermo Sánchez Mateos and Erica Salivioli have been awarded with a FPI-UAH contract to carry out their doctoral thesis at the UAH.
Congratulations 😊!
Marta Durán, Juan José Vaquero, Mercedes Griera, Sergio de Frutos, Diego Rodríguez Puyol, Javier García Marín*
La presente invención se refiere a nuevos compuestos que actúan como ligandos de la quinasa ligada a integrinas (del inglés ILK), facilitando la polimerización del citoesqueleto de actina y favoreciendo así el tráfico de receptores celulares desde los compartimentos intracelulares a la superficie de las células. Por este motivo, pueden ser utilizados en enfermedades como la diabetes mellitus tipo 2 o la diabetes insípida nefrogénica y, por extensión, en cualquier enfermedad en la que las alteraciones fisiopatológicas sean debidas, total o parcialmente, a un transporte transmembrana disminuido como las tubulopatías renales originadas por una pérdida de transportadores.
No: P202530222 (14/03/2025)
Clara Mañas, Estíbaliz Merino*
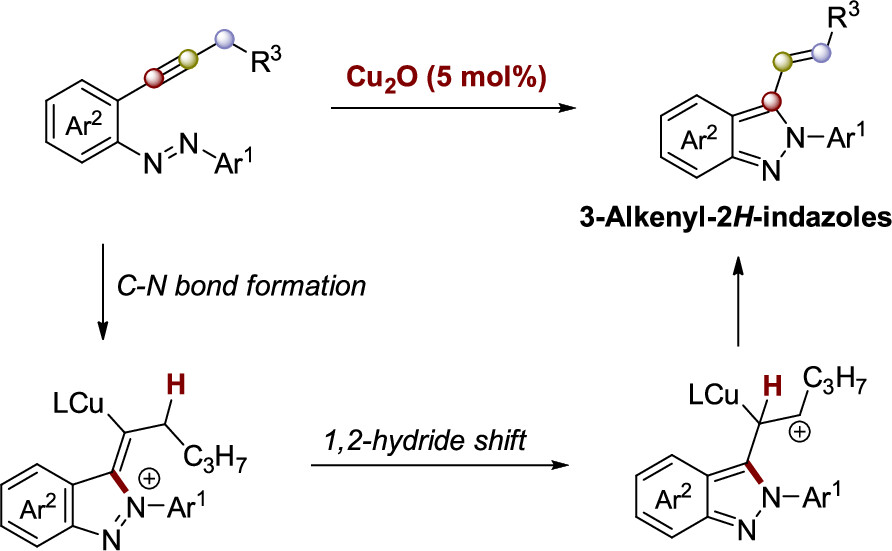
We disclose the intramolecular synthesis of 3-alkenyl-2H-indazoles from 2-alkynylazobenzenes, promoted by a dual catalysis using AuCl3 and a ruthenium photocatalyst under visible light irradiation. This reaction proceeds through hydroamination of the alkynyl fragment. The yields are influenced by electronic factors. Control experiments suggest that both radical and polar mechanisms operate in parallel. This transformation involves C−N bond formation and a 1,2-hydride shift. Additionally, derivatization was performed to demonstrate the versatility of this methodology..
Adv. Synth. Cat. 2025
DOI: 10.1002/adsc.202400990
Clara Mañas, Juan Herrero-Bordieu, Estíbaliz Merino*
A copper-catalyzed intramolecular synthesis of 3-alkenyl-2H-indazoles from 2-alkynylazobenzenes is described. The reaction proceeds in a single step via C–N bond formation and a subsequent 1,2-hydride shift, affording products in high yields. DFT calculations suggest the 1,2-hydride shift as the rate-determining step. Further derivatization enables functionalization of the 3-alkenyl-2H-indazoles.
J. Org. Chem. 2024
DOI: 10.1038/10.1021/acs.joc.4c02144
Clara Mañas, Belén Ibarra, Estíbaliz Merino*
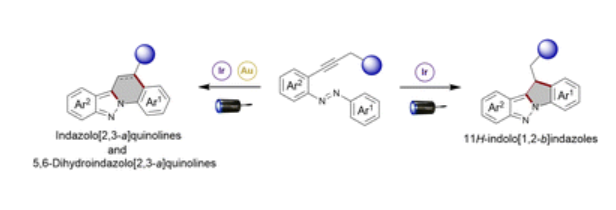
The development of regiodivergent methods that allow access to different structures from a single substrate through intramolecular processes is crucial for accelerating new molecule discovery, as well as making processes more sustainable and efficient in terms of waste production and economy. In this study, we report a novel regiodivergent cyclization procedure to access two distinct azapolyaromatic regioisomers from 2-alkynylazobenzenes. The key to achieving this regiodivergence lies in the presence or absence of a gold catalyst. The irradiation with visible light of 2-alkynylazobenzenes in the presence of a Ir photocatalyst affords 11H-indolo[1,2-b]indazoles, whereas under similar conditions with AuCl3, indazolo[2,3-a]quinolines are produced. Control experiments and DFT calculations suggest that both transformations operate through different reaction mechanisms: the formation of 11H-indolo[1,2-b]indazoles involves a radical mechanism, whereas the formation of indazolo[2,3-a]quinolines appears to proceed predominantly through a polar mechanism. This transformation enables the one-step conversion of simple 2-alkynylazobenzenes into diverse azapolyaromatic structures via an intramolecular visible light-promoted process, holding significant potential for new nitrogenated heterocycles.
Org. Chem. Front. 2024
DOI: 10.1038/s41929-024-01204-6
Dr. Ignacio Colomer, Tenured Scientist, at Organic Chemistry Institute (IQOG-CSIC), (Madrid)
Selective functionalization of alkenes using Hexafluoroisopropanols
Room 2.1, School of Pharmacy, UAH. February 07h, 12.15.
Belén Lerma-Berlanga, Francesco Orlando, Estíbaliz Merino, Antonio Leyva-Pérez*
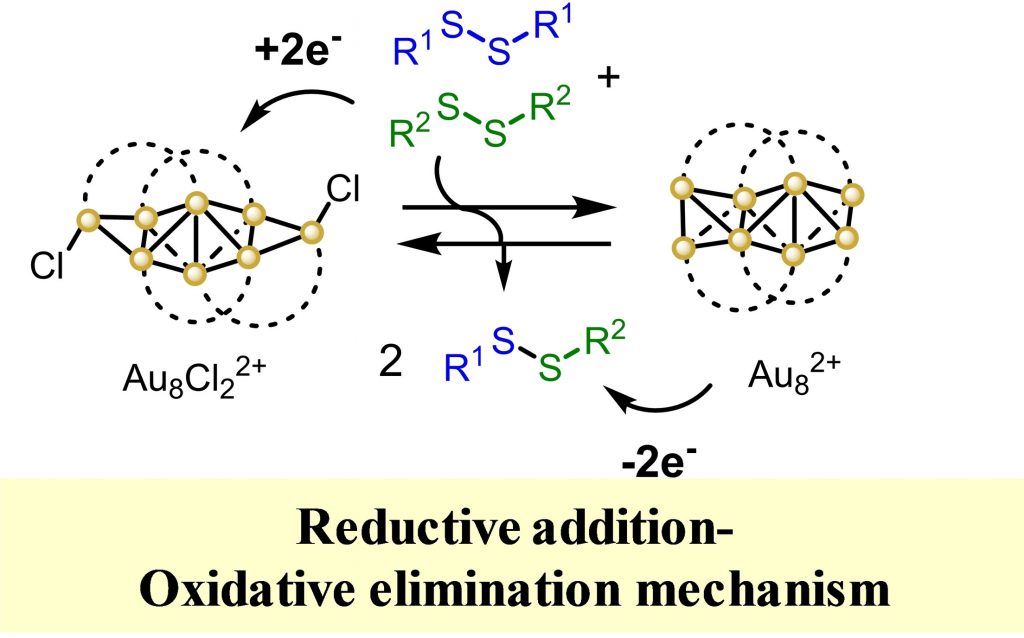
A plethora of industrial and academic organic chemical reactions are catalyzed by the well-known oxidative addition-reductive elimination (OARE) mechanism. However, the micro-reversed counterpart, i. e. the reductive addition-oxidative elimination (RAOE) mechanism, has to our knowledge not been reported yet in a catalytic manner. Here we show that a gold cluster can act as homogeneous catalyst capable of triggering a reductive addition step with disulfides, manage the incoming electrons and finally execute an oxidative elimination step, to perform the disulfide metathesis reaction. These findings suggest that not only new organic reactions but also other previously reported processes catalyzed by metal clusters may operate through the RAOE mechanism.
ChemCarChem 2024
DOI: 10.1038/s41929-024-01204-6
Estíbaliz Merino
Typically, active acyl intermediates are quenched with nucleophiles to complete carbonylation. Now, a visible-light-induced radical relay enables CO insertion and selective (hetero)aryl group migration without nucleophiles.
Nat. Cat. 2024.
DOI: 10.1038/s41929-024-01204-6
On March 2025, new students of the Master’s in Drug Discovery (UCM, UAH, CEU) have joined our group. During these months they will be carry out their Master’s Thesis Projects within the Biological Chemistry Group at the University of Alcalá in the Department of Organic and Inorganic Chemistry:
During the time with us, they will have access to state-of-the-art facilities and will work alongside experienced researchers and members of the group.
We wish you all a successful and rewarding experience and extend a warm welcome!
The University of Alcalá has awarded the Best Patent Award 2024 to two members of the Biological Chemistry Research Group, Dr. David Sucunza and Prof. Juan José Vaquero. The award recognizes their work and the research in the patent: Soluciones para diálisis peritoneal que contienen un flavonoide natural como agente osmótico (WO2024008987).
The patent, registered in collaboration with CSIC, claims for solutions for peritoneal dialysis containing a natural flavonoid as an osmotic agent. The present invention refers to peritoneal dialysis solutions that comprise troxerutin or vitamin P4 as an osmotic agent. The invention also refers to the procedure for preparing said solutions and to the novel use of troxerutin.
Congrats!!
