Publications > Coppola et al
Remote aryl cyanation via isocyanide-cyanide rearrangement on tosylmethyl isocyanide derivatives.
Departamento de Quimica Organica y Quimica Inorganica, Universidad de Alcala, 28871 - Alcala de Henares, Madrid, Spain.
Abstract
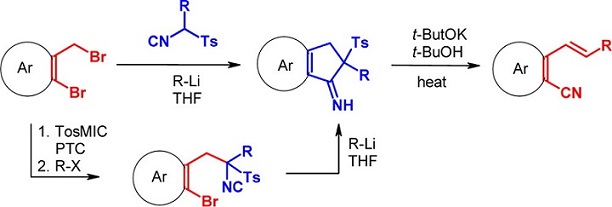
The reaction of alkyl tosylmethyl isocyanides and 2-bromobenzyl bromides in the presence of t-BuLi gives rise to a cascade reaction to give unexpected 2-substituted 2,3-dihydro-1H-indenimines which, upon treatment with t-BuOK, rearrange to 2-vinylbenzonitriles in high overall yields. This simple procedure represents a new approach to the synthesis of aromatic nitriles via isocyanide-cyanide interconversion.