Synthesis, Functionalization, and Optical Properties of 1,2-Dihydro-1-aza-2-boraphenanthrene and Several Highly Fluorescent Derivatives
Alberto Abengózar, Patricia García-García, David Sucunza, Diego Sampedro, Adrián Pérez-Redondo, and Juan J. Vaquero
Org. Lett., Article ASAP
DOI: 10.1021/acs.orglett.9b00448
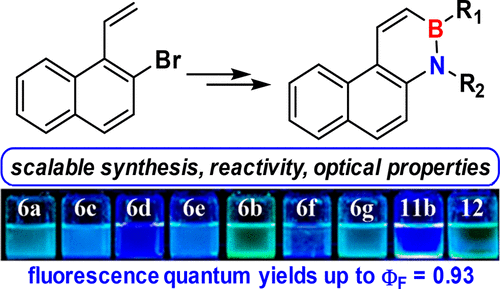
Previously unknown 1,2-dihydro-1-aza-2-boraphenanthrene has been synthesized in only three steps from 2-bromo-1-vinylnaphthalene. The reactivity of this new BN-phenanthrene, and of several substituted derivatives, has been tested against bromine and organolithium compounds. Bromination proceeded with complete regioselectivity, affording bromo-substituted compounds suitable for further functionalization via cross-coupling reactions. This new family of BN-phenanthrenes exhibits a substantial increase in the quantum yield (up to ϕF = 0.93) with respect to phenanthrene.
