Publications > Garre et al
Regiodivergent Electrophilic Cyclizations of Alkynylcyclobutanes for the Synthesis of Cyclobutane-Fused O-Heterocycles.
1. Departamento de Quimica Organica y Quimica Inorganica, Campus Cientifico-Tecnologico, Facultad de Farmacia , Universidad de Alcala (IRYCIS) , Autovia A-II, Km 33.1 , 28805 Alcala de Henares , Madrid , Spain. 2. Departamento de Quimica Organica e Inorganica, Instituto Universitario de Quimica Organometalica "Enrique Moles" , Universidad de Oviedo , C/Julian Claveria, 8 , 33006 Oviedo , Spain.
Abstract
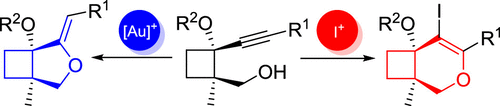
Cyclobutane-fused dihydropyrans and methylenetetrahydrofurans are highly interesting cores found in numerous natural products. Both these cores are selectively prepared from a common alkynylcyclobutane precursor bearing an appended hydroxyl group herein. Thus, cyclobutane-fused dihydropyrans can be obtained by a selective 6-endo-dig iodocyclization, whereas gold-catalyzed 5-exo-dig cycloisomerization provides a bicyclic core containing a methylenetetrahydrofuran moiety as major product. Several cyclobutane-fused O-heterocycles with diverse substituents are synthesized following the reported methodology.