Development of new synthetic methodologies
The development of new methodologies that allow the selective and efficient synthesis of complex molecules from simple reagents is one of the fundamental objectives of Organic Chemistry. Within this broad field of work, our research group focuses its interest on the following strategies: 1) applications of tosylmethylisonitrile (TosMIC) as a fundamental reagent in the formation of six-member nitrogenous heterocycles, 2) processes of C-X bond formation catalyzed by palladium complexes and 3) cycloisomerization of unsaturated systems (enines, alkynes, allenes…) by means of catalytic processes or halocylation reactions initiated by electrophilic activation of multiple C-C bonds.
• Use of TosMIC in the synthesis of nitrogenous heterocycles
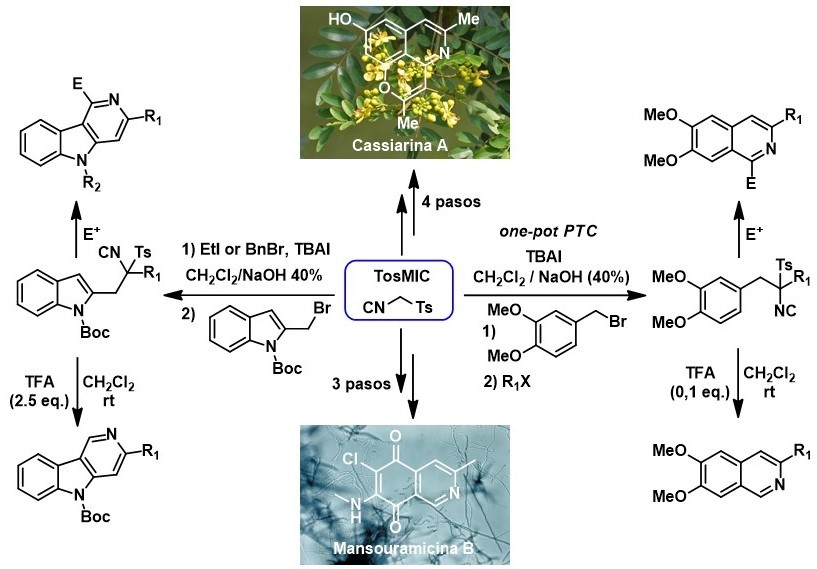
Due to the interesting properties of Tosilmetilisonitrilo (TosMIC) from a synthetic point of view, our group has taken advantage of it for the formation of six-member nitrogenous heterocycles, an aspect that, in contrast to the synthesis of five-member heterocycles, has been little studied. As major achievements in this line, we have developed new methodologies for the synthesis of isoquinolines and γ-carbolines and these have been used in the total synthesis of Variolin B, Mansouramicin B and Cassiarin A alkaloids.
Recent publications:
Synthesis of Multisubstituted Pyridines by Heterocyclization of TosMIC Derivatives: Total Synthesis of Caerulomycins A and K.
José A García-García, José Luis Aceña, Patricia García-García, David Sucunza
1,2,3-Benzotriazine Synthesis by Heterocyclization of p-Tosylmethyl Isocyanide Derivatives.
Francisco Maqueda-Zelaya, José Luis Aceña, Estíbaliz Merino, Juan J. Vaquero, David Sucunza
gamma-Carboline Synthesis by Heterocyclization of TosMIC Derivatives.
Sara Gutierrez, David Sucunza, Juan J Vaquero
Synthesis of 1-Substituted Isoquinolines by Heterocyclization of TosMIC Derivatives: Total Synthesis of Cassiarin A.
Sara Gutierrez, Anna Coppola, David Sucunza, Carolina Burgos, Juan J Vaquero
Isoquinoline synthesis by heterocyclization of tosylmethyl isocyanide derivatives: total synthesis of mansouramycin B.
Anna Coppola, David Sucunza, Carolina Burgos, Juan J Vaquero
• Development of catalytic processes of C-X bond formation
The most recent contribution in this field is the development of a general, efficient and functional group-tolerant methodology for the preparation of alkyl thioethers through the cross-coupling of alkyl halides and pseudo-halides with thiols.

Recent publications:
– General Synthesis of Alkenyl Sulfides by Palladium-Catalyzed Thioetherification of Alkenyl Halides and Tosylates.
N. Velasco, C. Virumbrales, R. Sanz, S. Suárez-Pantiga, M. A. Fernández-Rodríguez
Org. Lett. 2018, 20, 2848-2852. DOI
• Cycloisomerization processes of unsaturated systems promoted by electrophilic activation
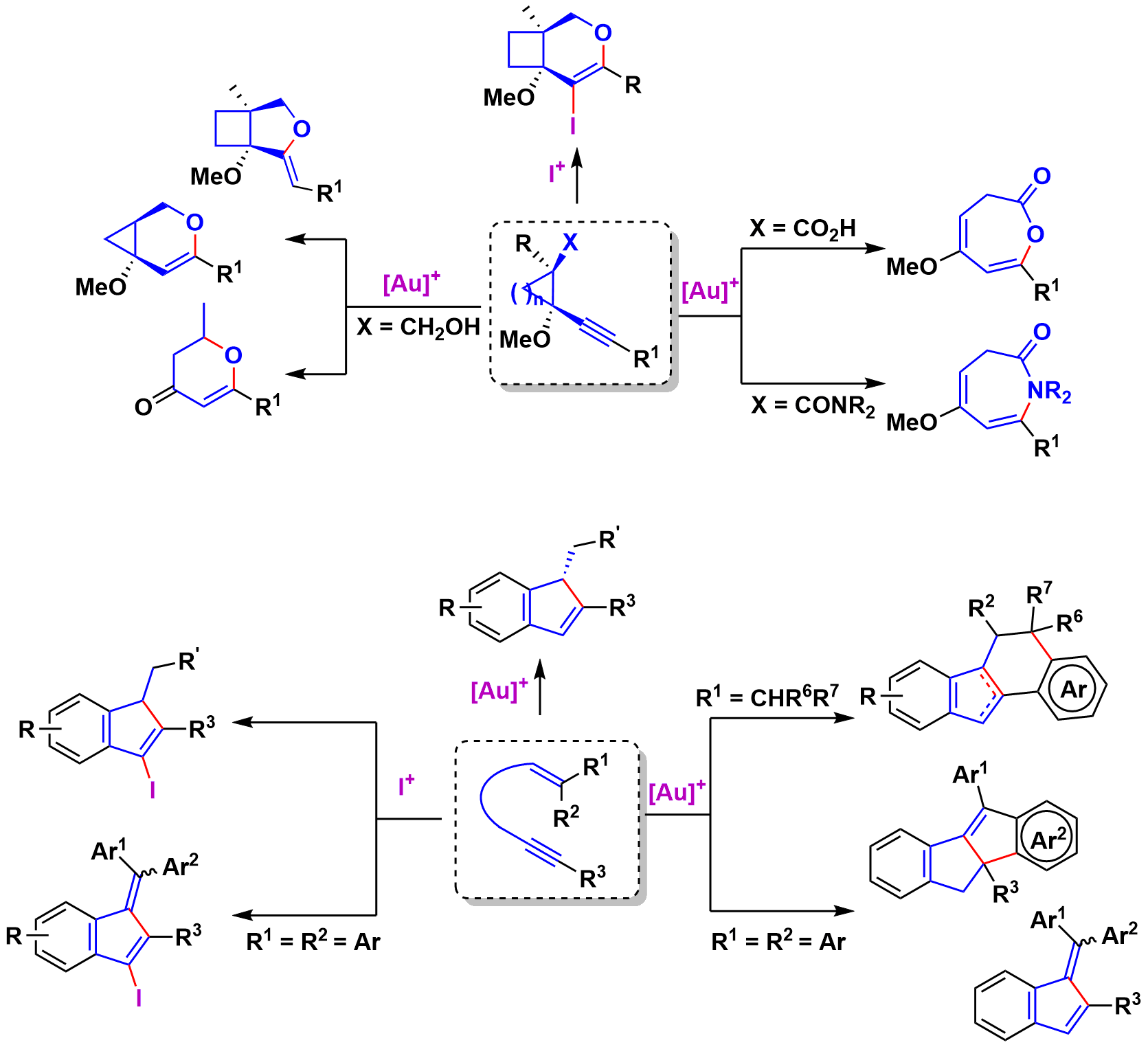
In this area in recent years we have described, in collaboration with Prof. E. Aguilar of the University of Oviedo and R. Sanz of the University of Burgos, the selective synthesis of various (hetero)cyclic compounds by processes initiated by the intramolecular nucleophilic addition of olefins or heteronucleophils on alkynes activated by gold(I) complexes or electrophilic iodine reagents.
Recent publications:
Divergent heterocycle synthesis enabled by switchable reaction of azobenzenes with alkynes.
Clara Mañas, Estíbaliz Merino
Selective synthesis of 4,5-dihydropyrenes by a Brønsted acid-catalyzed.
Jaime Tostado, Lucía Sánchez-Jiménez, Manuel A Fernández-Rodríguez
Selective Construction of Diverse Polycyclic Compounds by Electrophilic Cyclizations of Biaryl-Embedded 1,7-Enynes.
Lucía Sánchez-Jiménez, Patricia García-García, Manuel Á. Fernández-Rodríguez
Divergent synthesis of two polycyclic frameworks containing tricyclic bridgehead carbon centers by gold-catalyzed cycloisomerization of o-cyclopropylidenemethyl-o′-alkynylbiaryls.
Total Synthesis of Laetevirenol A via Regioselective Gold-Catalyzed and Acid-Promoted Cyclizations /
Ana Milián, Lucía Sánchez-Jiménez, Jaime Tostado, Juan J. Vaquero, Manuel A. Fernández-Rodríguez, Patricia García-García
Gold-catalyzed endo-selective cyclization of alkynylcyclobutanecarboxamides: synthesis of cyclobutane-fused dihydropyridones.
M Soledad Garre, Guillermo G Otárola, Estíbaliz Merino, David Sucunza, Enrique Aguilar, M Teresa Quirós, Juan J Vaquero, Patricia García-García
Selective Synthesis of Phenanthrenes and Dihydrophenanthrenes via Gold-Catalyzed Cycloisomerization of Biphenyl Embedded Trienynes.
Ana Milián, Patricia García-García, Adrián Pérez-Redondo, Roberto Sanz, Juan J Vaquero, Manuel A Fernández-Rodríguez
Gold-Catalyzed Synthetic Strategies towards Four-Carbon Ring Systems.
Guillermo G Otarola, Juan J Vaquero, Estíbaliz Merino, Manuel A Fernández-Rodríguez
Regiodivergent Electrophilic Cyclizations of Alkynylcyclobutanes for the Synthesis of Cyclobutane-Fused O-Heterocycles.
M Soledad Garre, David Sucunza, Enrique Aguilar, Patricia Garcia-Garcia, Juan J Vaquero
Gold(i)-catalyzed diastereoselective synthesis of 1-α-oxybenzyl-1H-indenes.
Cintia Virumbrales, Samuel Suárez-Pantiga, Marta Solas, Manuel A Fernández-Rodríguez, Roberto Sanz
Synthesis of Functionalized 1H-Indenes and Benzofulvenes through Iodocyclization of o-(Alkynyl)styrenes.
Patricia García-García, Ana M Sanjuán, Muhammad A Rashid, Alberto Martínez-Cuezva, Manuel A Fernández-Rodríguez, Félix Rodríguez, Roberto Sanz
Gold-Catalyzed Cycloisomerizations of Functionalyzed Cyclopropyl Alkynes: the Cases of Carboxamides and Alcohols
Jesús M. Fernández-García, Hugo A. Garro, Laura Fernández-García, Patricia García-García, Manuel A. Fernández-Rodríguez, Isabel Merino, Enrique Aguilar
1,3-Dien-5-ynes: Versatile Building Blocks for the Synthesis of Carbo- and Heterocycles.
Enrique Aguilar, Roberto Sanz, Manuel A Fernández-Rodríguez, Patricia García-García
Formal %4+1% Cycloadditions of β,β-Diaryl-Substituted ortho-(Alkynyl)styrenes through Gold(I)-Catalyzed Cycloisomerization Reactions.
Ana M Sanjuán, Cintia Virumbrales, Patricia García-García, Manuel A Fernández-Rodríguez, Roberto Sanz
Gold-catalyzed synthesis of oxepinones: an experimental mechanistic evidence
Eva M. Otero, Jesús M. Fernández-García, Manuel A. Fernández-Rodríguez, Enrique Aguilar
Gold(I)-catalyzed cycloisomerizations and alkoxycyclizations of ortho-(alkynyl)styrenes.
Ana M Sanjuán, Muhammad A Rashid, Patricia García-García, Alberto Martínez-Cuezva, Manuel A Fernández-Rodríguez, Félix Rodríguez, Roberto Sanz



