https://quibio.web.uah.es/group/
y actualice sus enlaces.
Publicaciones > Señorans et al
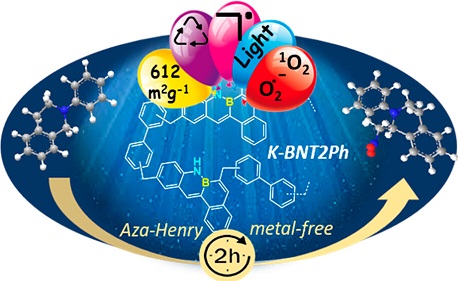
Hyper-Cross-Linked Porous Polymer Featuring B-N Covalent Bonds (HCP-BNs): A Stable and Efficient Metal-Free Heterogeneous Photocatalyst.
1. Department of Frontiers in Materials Chemistry, Instituto de Ciencia de Materiales de Madrid (ICMM-CSIC), Sor Juana Inés de la Cruz, 3, Cantoblanco, Madrid 28049, Spain. 2. Universidad de Alcalá (IRYCIS), Departamento de Química Orgánica y Química Inorgánica, Instituto de Investigación Química "Andrés M. del Río" (IQAR), Campus Científico-Tecnológico, Facultad de Farmacia, Autovía A-II, Km 33.1, 28805-Alcalá de Henares, Madrid, Spain.
Abstract
The first example of a porous polymer containing B-N covalent bonds, prepared from a tetraphene B-N monomer and biphenyl as a comonomer, is reported. It was prepared using the solvent knitting strategy, which allows the connection between the aromatic rings of the two monomers through methylene groups provided by an external cross-linking agent. The new polymer exhibited micromeso porosity with an S(BET) of 612 m(2)/g, high thermal stability, and potential properties as a heterogeneous photocatalyst, since it is very active in the aza-Henry coupling reaction (>98% of conversion and selectivity). After the first run, the catalyst improves its photocatalytic activity, shortening the reaction time to only 2 h and maintaining this activity in successive runs. The presence of a radical in this structure that remains stable with successive runs makes it a new type of material with potential applications as a highly stable and efficient photocatalyst.