La página a la que intenta acceder ha sido trasladada a otra dirección.
https://quibio.web.uah.es/
Por favor, espere a ser redirigido o pulse en el enlace anterior.
The page you are trying to access has been moved to another address.
https://quibio.web.uah.es/
Please wait to be redirected or click on the link above.
https://quibio.web.uah.es/group/
y actualice sus enlaces.
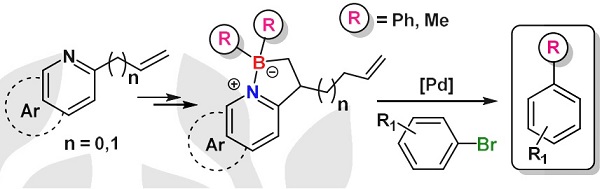
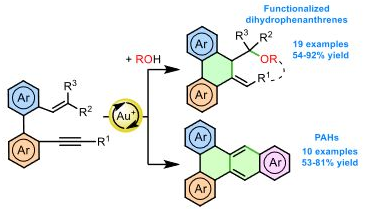
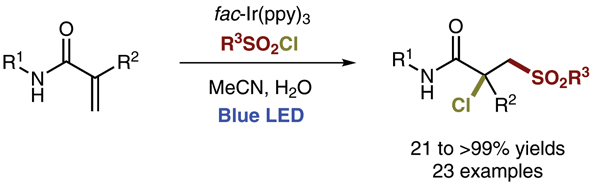
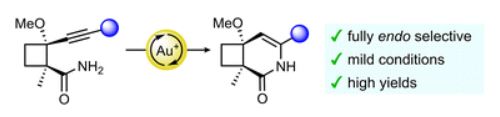
Gold-catalyzed endo-selective cyclization of alkynylcyclobutanecarboxamides: synthesis of cyclobutane-fused dihydropyridones.
1. Universidad de Alcalá (IRYCIS), Departamento de Química Orgánica y Química Inorgánica, Instituto de Investigación Química "Andrés M. del Río" (IQAR), Campus Científico-Tecnológico, Facultad de Farmacia, Autovía A-II, Km 33.1, 28805-Alcalá de Henares, Madrid, Spain. 2. Departamento de Química Orgánica e Inorgánica, Instituto Universitario de Química Organometálica "Enrique Moles", Universidad de Oviedo, C/Julián Clavería, 8, 33006 Oviedo, Spain.
a. juanjose.vaquero@uah.es b. juanjose.vaquero@uah.es c. juanjose.vaquero@uah.es d. juanjose.vaquero@uah.es e. juanjose.vaquero@uah.es f. juanjose.vaquero@uah.es g. juanjose.vaquero@uah.es
Abstract
Cyclobutane-fused dihydropyridones can be efficiently synthesized by a completely endo-selective gold-catalyzed cyclization of alkynylcyclobutanes bearing an appended amide, which proceeds under mild conditions. The observed selectivity, which is reversed from that previously observed for the cyclization of related alcohols and acids, is supported by DFT calculations.





 orcid.org/0000-0002-7953-4798.
orcid.org/0000-0002-7953-4798.