Esta versión de nuestra web ya no se mantiene actualizada.
Por favor, visite nuestra web operativa en
https://quibio.web.uah.es/group/
y actualice sus enlaces.
https://quibio.web.uah.es/group/
y actualice sus enlaces.
¡Gracias!
Chiral arylsulfinylamides as reagents for visible light-mediated asymmetric alkene aminoarylations
Cédric Hervieu, Mariia S. Kirillova, Yawen Hu, Sergio Cuesta-Galisteo, Estíbaliz Merino,* Cristina Nevado
Nat. Chem. 2024, Accepted Articles
DOI: 10.1038/s41557-023-01414-8
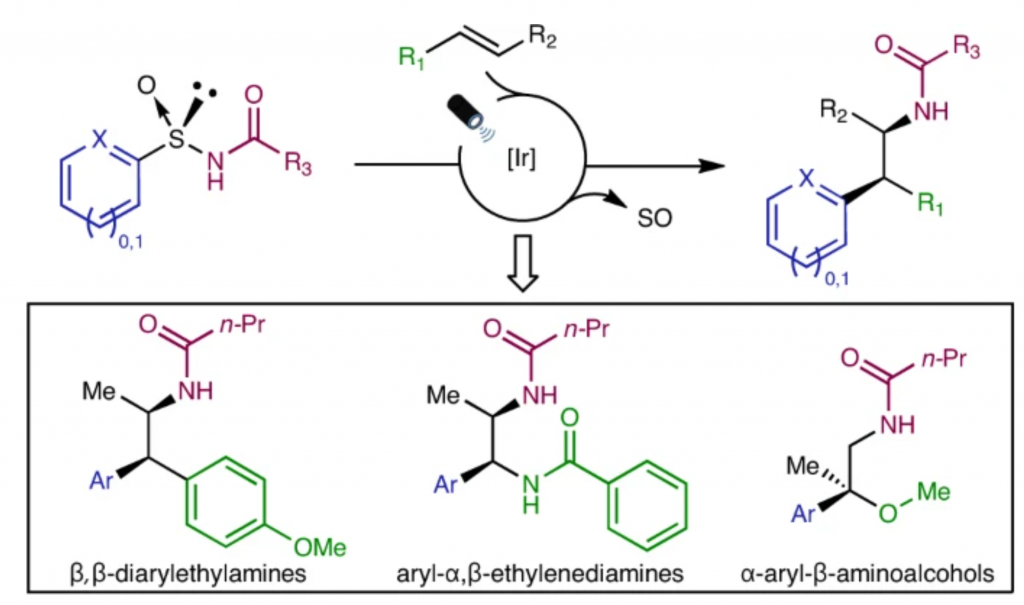
| Two- or one-electron-mediated difunctionalizations of internal alkenes represent straightforward approaches to assemble molecular complexity by the simultaneous formation of two contiguous Csp3 stereocentres. Although racemic versions have been extensively explored, asymmetric variants, especially those involving open-shell C-centred radical species, are much more limited both in number and scope. Here we describe enantioenriched arylsulfinylamides as all-in-one reagents for the efficient asymmetric, intermolecular aminoarylation of alkenes. Under mild photoredox conditions, nitrogen addition of the arylsulfinylamide onto the double bond, followed by 1,4-translocation of the aromatic ring, produce, in a single operation, the corresponding aminoarylation adducts in enantiomerically enriched form. The sulfinyl group acts here as a traceless chiral auxiliary, as it is eliminated in situ under the mild reaction conditions. Optically pure β,β-diarylethylamines, aryl-α,β-ethylenediamines and α-aryl-β-aminoalcohols, prominent motifs in pharmaceuticals, bioactive natural products and ligands for transition metals, are thereby accessible with excellent levels of regio-, relative and absolute stereocontrol. |