https://quibio.web.uah.es/group/
y actualice sus enlaces.
Dra. Isabel Rozas, Full Professor en la School of Chemistry of the Trinity College (Dublin)
A dónde puede llevarte elegir el ADN como diana biológica
Aula 0.6, Edificio Polivalente, UAH. January 11st, 16.00.
On-line: Rozas talk

Ana Milián, Lucía Sánchez-Jiménez, Jaime Tostado, Juan J. Vaquero, Manuel A. Fernández-Rodríguez*, Patricia García-García*
Adv. Synth. Catal. 2023, Accepted Articles
DOI: 10.1002/ejoc.202300535
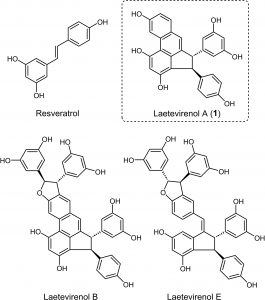
| The total synthesis of Laetevirenol A, a natural product with antioxidant activity, has been achieved. A gold-catalyzed cycloisomerization of an o-alkenyl-o’-alkynylbiphenyl has been used as the key step for the construction of the phenanthrene moiety present in Laetevirenol A. Several studies in model substrates have been carried out to unveil the effect of substituents in different locations in the outcome of this cyclization, which allowed the design of an appropriate precursor for the fundamental gold-catalyzed cycloisomerization. The suitably functionalized phenanthrene intermediate obtained in this key step could be further transformed into Laetevirenol A via a Friedel-Crafts cyclization, which also turned out to be dependent on the nature of the substituents. Finally, Laetevirenol A was obtained in 10 steps from commercially available substrates, with a 20% global yield. |

Senior Research Fellow

Responsable del grupo
Catedrático
 orcid.org/0000-0002-3820-9673
orcid.org/0000-0002-3820-9673

Catedrática

Profesor Titular

Profesor Ayudante Doctor
 orcid.org/0000-0002-1777-8199
orcid.org/0000-0002-1777-8199

Profesor Titular

Profesor Titular

Contratada “Ramón y Cajal”

Profesor Ayudante Doctor

Becario FPU Ministerio de Educación y Formación Profesional

Contratada predoctoral FPI MINECO 2015

Becario FPU MINECO 2017-Actualidad

Becaria FPI Universidad de Alcalá (2016-2020)

Becaria FPI Universidad de Alcalá (2017-2021)

Contrato RedInRen en Universidad Alcalá (2018)

Contrato RedInRen en Universidad Alcalá (2018)

Garantía Juvenil – Estudiante Predoctoral

Contrato Proyecto. Dpto. Química Orgánica y Química Inorgánica



![]()

Relevance of the Fanconi anemia pathway in the response of human cells to trabectedin.
Division of Hematopoiesis and Gene Therapy Program, Centro de Investigaciones Energeticas, Medioambientales y Tecnologicas, Avenida Complutense 22, 28040 Madrid, Spain.
Abstract
Trabectedin (Yondelis; ET-743) is a potent anticancer drug that binds to DNA by forming a covalent bond with a guanine in one strand and one or more hydrogen bonds with the opposite strand. Using a fluorescence-based melting assay, we show that one single trabectedin-DNA adduct increases the thermal stability of the double helix by >20 degrees C. As deduced from the analysis of phosphorylated H2AX and Rad51 foci, we observed that clinically relevant doses of trabectedin induce the formation of DNA double-strand breaks in human cells and activate homologous recombination repair in a manner similar to that evoked by the DNA interstrand cross-linking agent mitomycin C (MMC). Because one important characteristic of this drug is its marked cytotoxicity on cells lacking a functional Fanconi anemia (FA) pathway, we compared the response of different subtypes of FA cells to MMC and trabectedin. Our data clearly show that human cells with mutations in FANCA, FANCC, FANCF, FANCG, or FANCD1 genes are highly sensitive to both MMC and trabectedin. However, in marked contrast to MMC, trabectedin does not induce any significant accumulation of FA cells in G2-M. The critical relevance of FA proteins in the response of human cells to trabectedin reported herein, together with observations showing the role of the FA pathway in cancer suppression, strongly suggest that screening for mutations in FA genes may facilitate the identification of tumors displaying enhanced sensitivity to this novel anticancer drug.
Antitumor activity, X-ray crystal structure, and DNA binding properties of thiocoraline A, a natural bisintercalating thiodepsipeptide.
Departamento de Farmacologia, Universidad de AlcalA, E-28871 Madrid, Spain.
Abstract
The marine natural product thiocoraline A displayed approximately equal cytotoxic activity at nanomolar concentrations in a panel of 12 human cancer cell lines. X-ray diffraction analyses of orthorhombic crystals of this DNA-binding drug revealed arrays of docked pairs of staple-shaped molecules in which one pendent hydroxyquinoline chromophore from each cysteine-rich molecule appears intercalated between the two chromophores of a facing molecule. This arrangement is in contrast to the proposed mode of binding to DNA that shows the two drug chromophores clamping two stacked base pairs, in agreement with the nearest-neighbor exclusion principle. Proof of DNA sequence recognition was obtained from both classical DNase I footprinting experiments and determination of the melting temperatures of several custom-designed fluorescently labeled oligonucleotides. A rationale for the DNA-binding behavior was gained when models of thiocoraline clamping a central step embedded in several octanucleotides were built and studied by means of unrestrained molecular dynamics simulations in aqueous solution.
Publicaciones > Martinez et al
Benzo[f]azino[2,1-a]phthalazinium cations: novel DNA intercalating chromophores with antiproliferative activity.
Departamento de Quimica Organica, Universidad de Alcala, 28871-Alcala de Henares, Madrid, Spain.
Abstract
New azaquinolizinium-type cations have been obtained from isochromane. The synthesis was completed over seven steps and included as the key feature an intramolecular Westphal condensation. This first example of the intramolecular process allowed the preparation of benzo[f]pyrido[2,1-a]phthalazinium and benzo[f]quino[2,1-a]phthalazinium salts, which were evaluated as DNA intercalators, DNA topoisomerase I inhibitors, and antiproliferative compounds. Both cationic systems behave as DNA intercalators and exhibit antiproliferative activity. The pentacyclic benzo[f]quino[2,1-a]phthalazinium cations also have an inhibitory effect on the catalytic activity of DNA topoisomerase I, without trapping of cleavage complexes. Structural characterization using density functional theory indicates that the fused ring systems are slightly nonplanar, and additional molecular modeling studies suggest a preferred orientation for the intercalating chromophores within a typical CpG or TpG intercalation site.