Esta versión de nuestra web ya no se mantiene actualizada.
Por favor, visite nuestra web operativa en
https://quibio.web.uah.es/group/
y actualice sus enlaces.
https://quibio.web.uah.es/group/
y actualice sus enlaces.
¡Gracias!
Publicaciones > Coppola et al
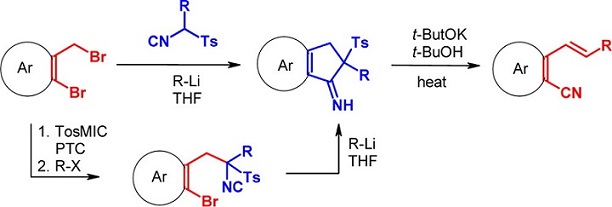
Remote aryl cyanation via isocyanide-cyanide rearrangement on tosylmethyl isocyanide derivatives.
Departamento de Quimica Organica y Quimica Inorganica, Universidad de Alcala, 28871 - Alcala de Henares, Madrid, Spain.
Abstract
The reaction of alkyl tosylmethyl isocyanides and 2-bromobenzyl bromides in the presence of t-BuLi gives rise to a cascade reaction to give unexpected 2-substituted 2,3-dihydro-1H-indenimines which, upon treatment with t-BuOK, rearrange to 2-vinylbenzonitriles in high overall yields. This simple procedure represents a new approach to the synthesis of aromatic nitriles via isocyanide-cyanide interconversion.