https://quibio.web.uah.es/group/
y actualice sus enlaces.
Publicaciones > Ramirez et al
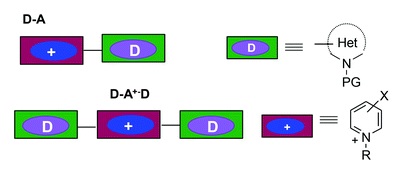
Synthesis of charged bis-heteroaryl donor-acceptor (D-A+) NLO-phores coupling (π-deficient-π-excessive) heteroaromatic rings.
1. Departamento de Química Orgánica y Química Inorgánica, Universidad de Alcalá, 28871-Alcalá de Henares, Madrid, Spain. 2. Department of Chemistry, University of Leuven, Celestijnenlaan 200 D, 3001 Leuven, Belgium. 3. Departamento de Quimica Analitica, Quimica Fisica e Ingenieria Quimica, Universidad de Alcalá, 28871 Alcalá de Henares, Madrid, Spain. 4. IES Matarraña, 44580 Valderrobres, Teruel, Spain.
Resumen
Charged chromophores based on heteroaromatic cations were prepared by reaction of alkylazinium salts with N-heteroarylstannanes under Stille conditions. This approach provides easy access to potential single donor D-A+ chromophores in which the acceptor moiety A+ is the pyridinium cation and the donors are different π-excessive N-heterocycles. The β hyperpolarizabilities were measured in hyper-Rayleigh scattering experiments and the experimental data are supported by a theoretical analysis that combines a variety of computational procedures, including density functional theory and correlated Hartree-Fock-based methods. In some chromophores, the absence of a bridge between donor and acceptor fragments increases the NLO properties. © 2013 The Royal Society of Chemistry.