https://quibio.web.uah.es/group/
y actualice sus enlaces.
Publicaciones > Milián et al
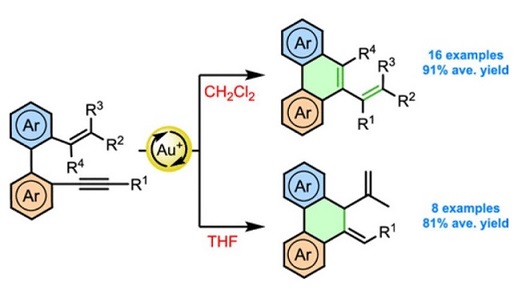
Selective Synthesis of Phenanthrenes and Dihydrophenanthrenes via Gold-Catalyzed Cycloisomerization of Biphenyl Embedded Trienynes.
1. Departamento de Quı́mica Orgánica y Quı́mica Inorgánica, Instituto de Investigación Quı́mica "Andrés M. del Rı́o" (IQAR). Universidad de Alcalá (IRYCIS). Campus Cientı́fico-Tecnológico, Facultad de Farmacia, Autovía A-II, Km 33.1, 28805 Alcalá de Henares, Madrid, Spain. 2. Área de Quı́mica Orgánica, Departamento de Quı́mica, Facultad de Ciencias, Universidad de Burgos, Pza. Misael Bañuelos s/n, 09001 Burgos, Spain.
Abstract
Readily available o'-alkenyl-o-alkynylbiaryls, a particular type of 1,7-enynes, undergo a selective cycloisomerization reaction in the presence of a gold(I) catalyst to give interesting phenanthrene and dihydrophenanthrene derivatives in high yields. The solvent used provokes a switch in the evolution of the gold intermediate and plays a key role in the reaction outcome.