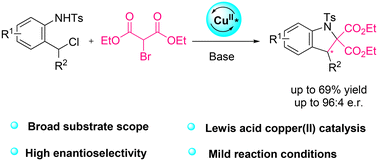
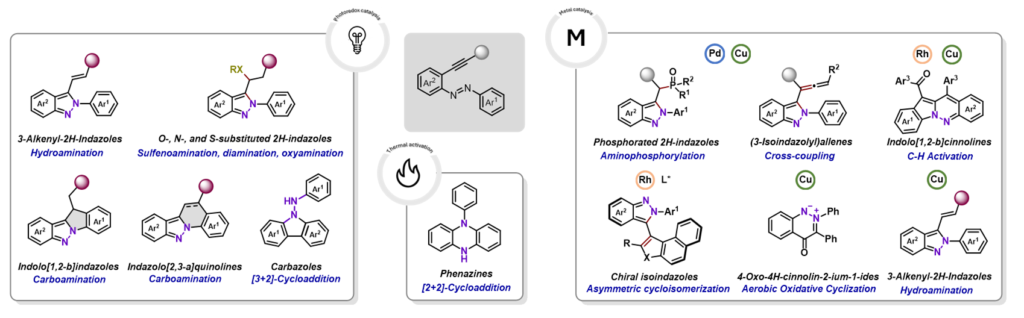
Aliphatic azo compounds as programmable nitrogen donors in alkyne-mediated heterocycle synthesis: Implications for medicinal chemistry
Clara Mañas, Estíbaliz Merino*
Nitrogen-containing heterocycles constitute the core of many approved drugs and clinical candidates, making efficient and predictable C–N bond construction a central objetive in medicinal chemistry. Aliphatic azo compounds, traditionally employed as radical initiators, have recently emerged as versatile programmable nitrogen donors, capable of transferring their nitrogen atoms directly into heterocyclic scaffolds. This review summarizes advances in the reactivity of azoaliphatic derivatives with alkynes, highlighting pathways where nitrogen atoms are retained in the final products and on their implications for drug delivery. Cycloaddition processes provide rapid access to privileged heterocycles such as pyrazoles and pyrroles, scaffolds that are well represented in marketed drugs and support early structure–activity relationship exploration. Complementary radical and carbenoid manifolds enable the formation of hydrazides, atropisomeric frameworks and rarer nitrogen–sulfur motifs, offering increased three-dimensionality and new vectors for tuning potency, selectivity and pharmacokinetic properties. Where available, representative case studies illustrate how these scaffolds have contributed to lead optimization, target selectivity or progression toward clinical evaluation. Beyond reactivity, this review critically evaluates scalability, operational robustness and sustainability to define when azo–alkyne methodologies are realistically applicable in medicinal chemistry workflows. Rather than presenting azo compounds as general-purpose reagents, we frame them as strategic nitrogen donors whose reactivity can be aligned with specific stages of the drug discovery pipeline. When used in this manner, azo–alkyne transformations enable efficient scaffold generation, late-stage diversification and access to underexplored chemical space relevant to modern medicinal chemistry.
Eur. J. Med. Chem. 2026
DOI: https://doi.org/10.1016/j.ejmech.2026.118648
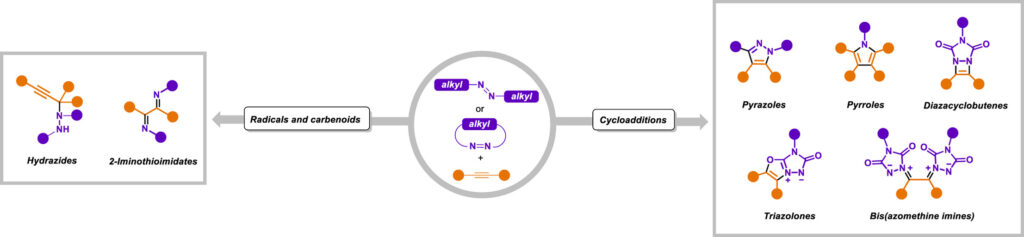

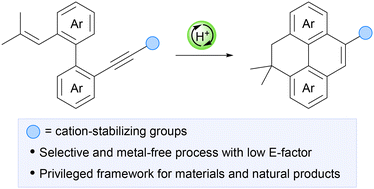
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: