Dr. García promoted to Full Professor at the UAH
Last friday, March 16th, Dr. García García was appointed as Full Professor at the UAH.
Congratulations Patricia!
Publications > Renedo et al
Advanced Synthesis & Catalysis. 2024; 366(9):2079 -2089.
Gold-Catalyzed Tandem Oxidation-Migration of 3-Propargyl Indoles: Synthesis of α-Indol-3-yl α,β-Unsaturated Carbonyls.
Lorena Renedo, Estela Álvarez, Marta Solas, Samuel Suárez-Pantiga, Manuel A. Fernández-Rodríguez, Roberto Sanz
Palabras clave: carbonyls; gold; homogeneous catalysis; indoles; oxidation
Resumen
No hay resumen
Dr. Merino promoted to Associate Professor at the UAH
Last friday, March 1st, Dr. Estibaliz Merino successfully passed her exam and was appointed as Associate Professor at the UAH.
Congratulations Estíbaliz!
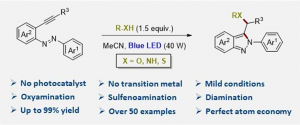
Visible Light-Mediated Heterodifunctionalization of Alkynylazobenzenes for 2H-Indazole Synthesis Clara Mañas and Estíbaliz Merino*
Org. Lett. 2024, ASAPacs.orglett.4c00097
We disclose the heterodifunctionalization of alkynylazobenzenes promoted exclusively by visible light in the absence of any transition metal and/or photocatalyst. This reaction features excellent regioselectivity on a broad variety of substrates with perfect atom economy. Alcohols, carboxylic acids, thiols, amides, heterocycles, and even water are suitable substrates for the promotion of the oxyamination, sulfenoamination, and diamination reactions. In this manner, biologically active indazole scaffolds can be rapidly assembled from alkyne feedstocks.
Javier Recio Ramos
Assistant Professor
2024-Present: Assistant Professor at Universidad Rey Juan Carlos.
2024: Postdoctoral researcher ‘Margarita Salas’, at Universidad de Alcalá (Prof. Manuel Ángel Fernández).
2022-2023: Postdoctoral researcher ‘Margarita Salas’ at CIB-CSIC (Spain) (Prof. Ana Martínez).
2021: Scientific Supervisor at Centro de Química Aplicada y Biotecnología (CQAB) UAH.
2020-2021: Postdoctoral researcher (ERC-associated project), Universität Hamburg (Germany), (Prof. Axel Jacobi von Wangelin).
2020 PhD in Medicinal Chemistry (FPI-UAH grant) at Universidad de Alcalá, (Prof. Julio Álvarez / Prof. Carolina Burgos).
2018: Predoctoral research stay, Universität Hamburg (Germany) (Prof. Axel Jacobi von Wangelin).
2015 Master in Química Fina by Universidad de Alcalá (Spain).
2014: Graduted (Chemistry) at Universidad de Alcalá (Spain).
FPI (project-associated), Universidad de Alcalá (2022-2023)
Master Organic Chemsitry , Universidad Autónoma de Madrid (2022-2023)
Graduated (Chemistry), Universidad Autónoma de Madrid (2018-2022)
Publications in the group
Shaping cycles with light: a regiodivergent approach to tetracyclic aza-aromatic compounds.
Clara Mañas, Belén Ibarra, Estíbaliz Merino
Elio Mollá Arroyo
FPI (project-associated), Universidad de Alcalá (2022-2023)
Master Organic Chemsitry , Universidad Autónoma de Madrid (2022-2023)
Graduated (Chemistry), Universidad Autónoma de Madrid (2017-2022)
Carmen Callejón Martínez
Project associate researcher Universidad de Alcalá (2022)
“Contrato Yo Investigo” researcher at Universidad de Alcalá (2021 2022)
Master Applied and Pharmacological Chemistry , Universidad de Castellón (2020/2021)
Graduated (Cehmistry) at the Universidad de Jaen (2019)
Guillermo Morales
Comunidad de Madrid Predoctoral Gran (2023)
Project associate researcher FIBioHRC Universidad de Alcalá (2022-2023)
Master Drug Discovery , Universidad Complutense (2021-2022)
Graduated (Pharmacy) at the Universidad de Alcalá (2017-2022)
Publications in the group
Visible-light-initiated metal-free C(sp(3)) -C(sp(3)) to C(sp(3)) -N conversion in homobenzylic sulfonamides with N-iodoimides.
Guillermo Morales-Ortega, Estíbaliz Merino, Javier Carreras
Sergio Torres
0000-0002-1003-519X
Project associate researcher FIBioHRC Universidad de Alcalá (2022-2023)
“Contrato Yo Investigo” researcher at Universidad de Alcalá (2021 2022)
Master Drug Discovery , Universidad Complutense (2021/2022)
Master in Industrial Chemistry and Introduction to the Chemical Research, Universidad Autónoma de Barcelona (2017-2018)
Graduated in Chemistry at the Universidad Autónoma de Barcelona (2013-2017)
Publications in the group
Synthesis of 3,4-Dihydroquinazolinones via Base-Promoted Formal %4 + 2% Cycloadditions.
Sergio Torres-Oya, Manuel A. Fernández-Rodríguez, Mercedes Zurro
Visible-light mediated synthesis of bicalutamide by regioselective hydroxysulfonylation of acrylamides.
Mercedes Zurro, Sergio Torres-Oya, Estíbaliz Merino
Visible-Light-Mediated Regioselective Chlorosulfonylation of Acrylamides.
Mercedes Zurro, Sergio Torres-Oya, Guillermo G. Otárola, Juan José Vaquero, Estíbaliz Merino
Posts navigation
Biological Chemistry Group