Visible Light-Mediated Heterodifunctionalization of Alkynylazobenzenes for 2H-Indazole Synthesis
Clara Mañas and Estíbaliz Merino*
Org. Lett. 2024, ASAP
DOI: acs.orglett.4c00097
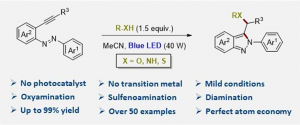
| We disclose the heterodifunctionalization of alkynylazobenzenes promoted exclusively by visible light in the absence of any transition metal and/or photocatalyst. This reaction features excellent regioselectivity on a broad variety of substrates with perfect atom economy. Alcohols, carboxylic acids, thiols, amides, heterocycles, and even water are suitable substrates for the promotion of the oxyamination, sulfenoamination, and diamination reactions. In this manner, biologically active indazole scaffolds can be rapidly assembled from alkyne feedstocks. |
