Synthesis of Seven- and Eight-Membered Rings by a Brønsted Acid Catalyzed Cationic Carbocyclization of Biphenyl Embedded Enynes
Jaime Tostado , Ana Milián, Juan J. Vaquero, and Manuel A. Fernández-Rodríguez*
Org. Lett. 2024, ASAP
DOI: acs.orglett.4c00647
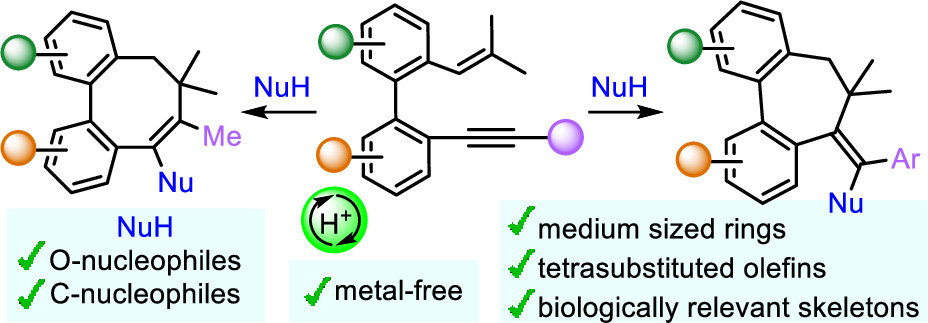
| A Brønsted acid catalyzed cyclization of o-alkenyl-o′-alkynylbiaryls for the synthesis of biologically relevant dibenzo-fused medium-sized rings has been developed. The outcome of the cyclization is determined by the nature of the substituent at the alkyne, with arenes favoring seven-membered rings and alkyl substituents producing eight-membered rings. These reactions proceed via a vinyl cation, which is captured by water and, notably, by C-nucleophiles, such as electron-rich (hetero)arenes. |
