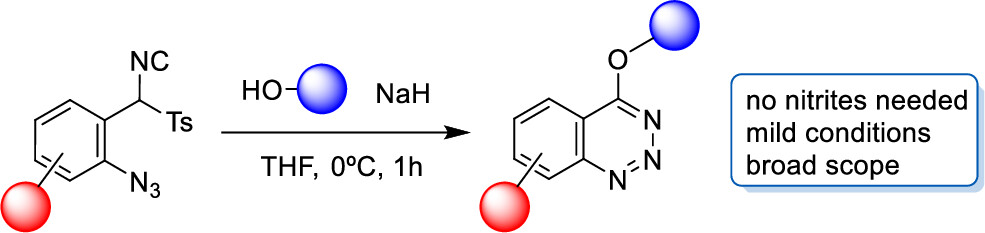
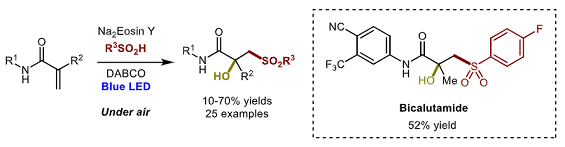
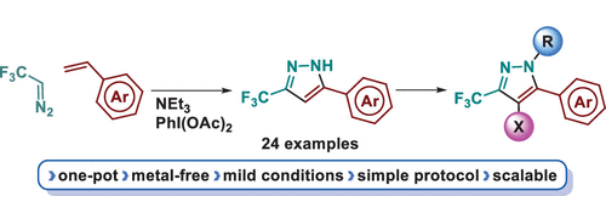
La página a la que intenta acceder ha sido trasladada a otra dirección.
https://quibio.web.uah.es/
Por favor, espere a ser redirigido o pulse en el enlace anterior.
The page you are trying to access has been moved to another address.
https://quibio.web.uah.es/
Please wait to be redirected or click on the link above.
https://quibio.web.uah.es/group/
y actualice sus enlaces.